Andrew Taylor, Professor of Ophthalmology, highlights research to understand the molecules that mediate ocular immune privilege so they can be adapted for chronic autoimmune uveitis
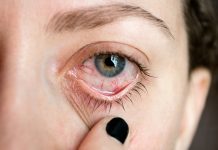
Current therapy uses steroids, which have been the standard approach for over six decades. The recent use of humanized antibodies, biologics, to suppress key cytokines in the inflammatory pathway is providing an alternative to steroids; however, like steroid therapy carry serious side-effects from susceptibility to infection to even death. Ironically steroid therapy carries the risk of sight-threating cataracts and glaucoma.
Nearly 17% of patients suffer chronic uveitis and are resistant to steroid therapy. This leaves them with months to years of finding the right concentration of a biological to manage uveitis with minimal side-effects. The objective of this therapy is to suppress inflammation to give the ocular tissue a chance to recover, and possibly reassert its normal anti-inflammatory mechanisms. Meanwhile, each episode of uveitis further diminishes vision leading to blindness and reduced quality-of-life.
One of the ultimate goals of autoimmune disease therapy is to find an approach that would return the immune system to treating our own tissues as self. Such a therapy would not only suppress inflammation; it would induce what could be considered hitting the reset button of the immune system to prevent recurrence of autoimmune disease. Studies into the mechanisms of ocular immune privilege are revealing molecules that have the potential to reset immunity. Not only can these molecules be used as therapy, but they are also well tolerated and are molecules naturally expected to be expressed in a healthy eye.
Originally, the concept of ocular immune privilege was a transplantation term defined by Sir Peter Medawar as a tissue site where incompatible grafts survive indefinitely. Today, the definition of immune privilege includes a tissue microenvironment that actively suppresses the induction of inflammation and promotes immune tolerance. Immune tolerance is where immune cells regulate the activity of other immune cells.
Immune privilege is mediated by molecules expressed on cell membranes and secreted by cells within the eye. The result is that for most of us we have a lifetime of vision not threated by inflammation and autoimmune disease. Our studies into these molecules of ocular immune privilege have demonstrated the role of several that hold a central role in ocular immune privilege and a role in immune regulation systemically.
One of these molecules is the neuropeptide alpha-melanocyte stimulating hormone (α-MSH) and its melanocortin receptors (MCr). This neuropeptide first described for its ability to regulate pigmentation has roles in metabolism, immunity, and general wellbeing. It and its receptors are highly conserved and are expressed in every animal. When we started studying the soluble molecules of ocular immune privilege, we had found that aqueous humour, the fluid that fills the anterior chamber of the eye, did not suppress T cell activity. It changed T cell activity from proinflammatory to anti-inflammatory.
Of the many molecules we found in aqueous humour that suppress inflammation, only α-MSH made T cells expected to mediate inflammation suppress inflammation. In addition, we found that α-MSH like aqueous humour makes other activated immune cells, like macrophages, to make anti-inflammatory cytokines and suppress inflammation.
Removal of α-MSH from aqueous humour eliminated the ability of aqueous humour to mediate anti-inflammatory activity. The results alone are enough to support melanocortin-based therapies to suppress uveitis; however, while we were studying the use of α-MSH in therapy more was discovered, suggesting that melanocortin-based therapy can promote immune tolerance, the necessary reset of immunity.
The best-used mouse model of autoimmune uveitis is called experimental autoimmune uveoretinitis (EAU). EAU has served as an important model in understanding the activity of immune cells in autoimmune disease, testing for new therapies, and understanding the mechanisms of immune privilege. One interesting feature of this model is that unlike in humans, mice resolve uveitis on their own without intervention with resistance to a recurrence of the disease. An important feature of post-EAU is the expansion of regulatory T (Treg) cells specific to antigens within the eye. We found that α-MSH mediates the expansion of these eye-specific Treg cells.
Moreover, we found that α-MSH through the melanocortin five receptor (MC5r) activates an antigen presenting cell (APC) that in a process called counter-conversion coverts T cells that would mediate autoimmune disease into T cells that suppress autoimmune disease. These T cells are called inducible Treg cells, and they protect against reactivation of EAU. Treatment of EAU with α-MSH accelerates recovery from EAU, induces the expansion of the inducible Treg cells, and protects retinal structure from inflammation.
The melanocortin-based therapy may very well reestablish ocular immune privilege. Even if α-MSH is one of many molecules of immune privilege with the ability to induce tolerance, our findings demonstrate that using the melanocortin pathway is potentially an effective therapeutic approach to treat autoimmune uveitis, and preserve vision.
The molecular mechanisms of ocular immune privilege are a wealth of potential therapies to be exploited to suppress uveitis. Our findings that activation of the melanocortin pathway through the neuropeptide α-MSH is not only essential for ocular immune privilege but that it can be used to suppress autoimmune uveitis. This is a new therapeutic approach using our body’s natural molecules of ocular immune privilege, in our studies the neuropeptide α-MSH, to change the behaviour of immune cells to suppress inflammation, and autoimmune uveitis.
In addition, there is a strong possibility that melanocortin-based therapy moves us closer to the ultimate goal of resetting immunity to prevent and stop chronic autoimmune uveitis.
Andrew W Taylor
Associate Dean of Research,
Professor of Ophthalmology
Boston University School of Medicine
Tel: +1 617 358 2311
awtaylor@bu.edu
http://www.bumc.bu.edu/ophthalmology/research-programs/andrew-w-taylor-phd/
*Please note: This is a commercial profile
Editor's Recommended Articles
-
Must Read >> New challenges for public health in the 21st century