A group of experts from the International Society for Biological and Environmental Repositories, (ISBER) shed light on the science of biobanking and its role in delivering modern and precision medicine
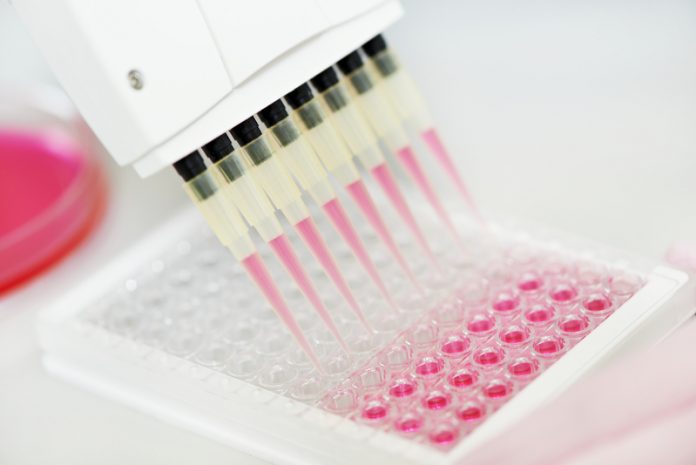
Biobanking is increasingly recognised as an essential activity underpinning scientific breakthrough in precision medicine and leading to new treatments(1). Biobanking is a collective term which describes the process by which biological samples (bodily fluid or tissue) and associated data are collected, annotated, transferred, stored and redistributed for future research in order to improve our understanding of health and disease. The last two decades have seen sustained growth in the creation of biobanks and as such, biobanks now exist in almost every country, university and medical research institute/hospital.
Precision medicine research is based on the analysis of samples with clinical data – and, because the associations are often weak, we need these samples in large quantities. The implication is clear: if more, well-characterised, high-quality samples are available through biobanks, the faster research will advance and impact upon the faster delivery of precision healthcare today. The systematic collection of human samples of high quality is a key element to the success of future treatments. Biobanks are now being relied upon to standardise tissue collection for improved science quality and to actually drive research.
Biospecimen research: Where biobanking becomes science
A new line of thinking is emerging in biobanking: the need to identify and develop indicators (biomarkers) to show if a given sample has the quality to be used with a certain technique. Before using the sample, a simple test can show its quality and usability and if during the lifetime of the sample, it was kept in the appropriate conditions. (2,3) Such biomarkers should allow researchers to compare analytical results from biologically identical samples, processed in different ways or stored for different periods of time. Biospecimen research is a new concept and often scientific journal editors refuse to consider such manuscripts for review, stating that the subject is not considered to be a priority. However, sample quality underpins the accuracy of all subsequent research done with those samples and can impact on the reproducibility and robustness of the results.
A number of governments globally, including the U.S. and the EU, are increasingly interested in this area on account of the lack of reproducibility in scientific research. ISBER is leading global discussions aimed at the identification of such biomarkers. ISBER has organised a number of Biospecimen Research Symposia (Luxembourg, February 2018; Berlin, February 2019) with global leaders in the field. As the technology in medical research develops at a fast pace, biobanks need to provide the evidence of the shelf-life or the fitness-for-purpose of the samples they supply.
Harmonisation of standards leads to scalability
The two most important aspects of a biobank are consistency and quality. The validity of the data generated by biobanked samples depends on their quality, which is, in turn, is dependent on the use of stringent standards in collecting these samples and delineating patient characteristics. A number of best practices and guidelines have been published over the last few years, such as the ISBER Best Practices 4th edition; (4) while at the end of 2018, the International Biobanking standards are expected to be published by the ISO TC276. (5) For the first time, these documents will be aligned, indeed, this development is expected to introduce a new level of harmonisation across the entire field. The expectation is that the introduction of these standards will allow for the scaling of the biobanking operations and the support of major research projects, both in academia and the pharmaceutical industry.
Innovative digital technologies
Biobanking is not only the collection of biological samples but also of related data which would allow the meaningful study of the collected tissue. The addedvalue for the collected samples is created by annotation, analysis and transformation of samples to other derivatives, such as genetic information. All these technological advancements will need to remain aligned with proper data governance and donor privacy protection. The management of human tissue in research, therefore, inevitably leads to a requirement to manage the data generated from laboratory investigations and linked clinical sources.
Aided by a continuous level of standardisation, new methods such as predictive analytics and artificial intelligence (AI) are slowly making inroads into biobanking. The interrogation of vast quantities of data from a large biobank can now be completed within a few weeks (even remotely) as opposed to months or years previously. The large size of many biobanks, coupled with the enormous potential of health records and other electronic health data, place this type of research in the forefront of making significant contributions to health care.
New capacities requiring new support mechanisms
This aspect of biobanking sustainability is critical for precision medicine research. The increased demands on the types of samples and processing provided by biobanks create increasing operating and financial pressures. Success cannot be gauged any longer by simple supply and demand principles and judged on the efficiency of the ability to move samples. (6) Instead, if we accept the fundamental truth that banked samples are sources of information, biobanks should be judged on their ability to generate knowledge, the discovery of clinical innovations and implementation of new practices that will ultimately lead to improved health outcomes for patients.
References
1 Biobanking Contributing to Breakthroughs in Rare Diseases http://news.isber.org/mtm_raredisease/
2 Betsou F, et al. (2013) Identification of evidence-based biospecimen quality control tools. J Mol Diagnostics; 15:3-16.
3 Betsou F and ISBER Biospecimen Science Working Group. (2016) Assays for qualification and quality stratification of clinical biospecimens used in research. Biopreserv Biobank; 14:398-409.
4 Simeon-Dubach D and Kozlakidis Z. (2018) New Standards and Updated Best Practices Will Give Modern Biobanking a Boost in Professionalism. Biopreserv Biobank; 16(1):1-2.
5 ISO/DIS 20387. Biotechnology-Biobanking-General requirements for biobanking (published 8th of August 2018).
6 Zhou L and Catchpoole D (2015) Spanning the genomics era: the vital role of a single institution biorepository for childhood cancer research over a decade. Transl Pediatr.; 4(2):93-106.
Zisis Kozlakidis
ISBER Past President
Head, Laboratory Services and Biobanking
International Agency for Research on Cancer (WHO), Lyon, France
David Lewandowski
ISBER President
Brooks Life Science Systems, Chelmsford, United States
Fay Betsou
Chief Scientific Officer,
Integrated Biobank of Luxembourg (IBBL)
International Society for Biological and Environmental Repositories
(ISBER)
Tel: +1 604 484 5693
Editor's Recommended Articles
-
Must Read >> The need for special education in biobanking
-
Must Read >> The growing need for innovation in biobanking
-
Must Read >> Smart Biobanking