Prof. Dr. Tanja Gaich, of the University of Konstanz – Department of Chemistry discusses new strategies in drug development and the challenges involved
Finding and designing new pharmaceuticals based on small organic molecules literally constitutes the search for the needle in the haystack. The first challenge thereby is to find the specific structure with the requested biological activity among myriads (~1060) of possible organic molecules. But not enough, this molecule also needs to meet certain standards with regards to metabolic stability, bioavailability, toxicology etc. mandated by the drug agencies. In the process of evolution – over millions of years – Nature has singled out so called “privileged structures” exhibiting all kinds of biological activities and uses them for its purposes (chemical communication; chemical defense etc.). These “privileged structures” e.g. natural products are produced by organisms of the animal, as well as the plant-kingdom. Ever since mankind has taken advantage of nature by what is known today as “ethno or traditional medicine”.
Traditionally, extracts, teas or pastes of these organisms containing these natural products have been used without the knowledge of their exact structure and mode of action. Nowadays, natural product researchers capitalise on the head-start Nature provides with these “privileged structures”, by identifying the exact molecular architecture of these natural products and understanding their mode of action, thereby enabling rational drug design. Yet, these investigations strongly depend on the actual availability and natural abundance of the investigated natural products. Extracts of organisms typically contain very low quantities (scale: mg/kg dry weight of organism) of natural products, making drug development based on isolated material from their natural producers virtually impossible. Synthetic access to natural products – independent of the natural producer – is therefore inevitable for the development of new drugs, since exploitation of the producing organisms would threaten them with extinction.
Challenges
Since the molecular architecture of natural products is very complex, their laboratory synthesis poses a formidable challenge for synthetic organic chemists and represents the cutting edge of the science of synthesis. Consequently, natural products remain an underexplored source in pharmaceutical industry. Nevertheless, around 32% of pharmaceuticals in the market to date are based on small molecules derived from natural products (Figure 1), underscoring their vital importance in drug design. What makes the application of natural products in drug development further challenging is the fact, that synthetic access to “derivatives” is extremely laborious and very often impossible. “Derivatives” constitute small structural changes introduced to the original molecular architecture by means of organic synthesis. These small changes of the original molecular structure are indispensable in drug development. Nature has designed natural products for its own purposes, which do not necessarily contain aspects of pharmacology in humans or meet the high approval standards of national drug agencies. Thus, “derivatisations” constitute an optimisation process of natural products with regard to these standards and requirements, and ultimately allow to bring the drug to the market. However, in conventional organic synthesis almost every small structural change (derivatisation) in the original molecular architecture requires a complete redesign of the synthetic route. In other words, a small change in molecular structure does not lead to an incremental change in the synthetic problem, but potentiates it. On the other hand, a big advantage of the complexity of natural product structures and opportunity for drug design, is the fact, that often “substructures” of less molecular complexity than the natural product itself already display the same biological activity. This reduces the complexity of the synthetic problem, only demanding a synthesis of a substructure of the molecule and its subsequent derivatisations.
Objectives
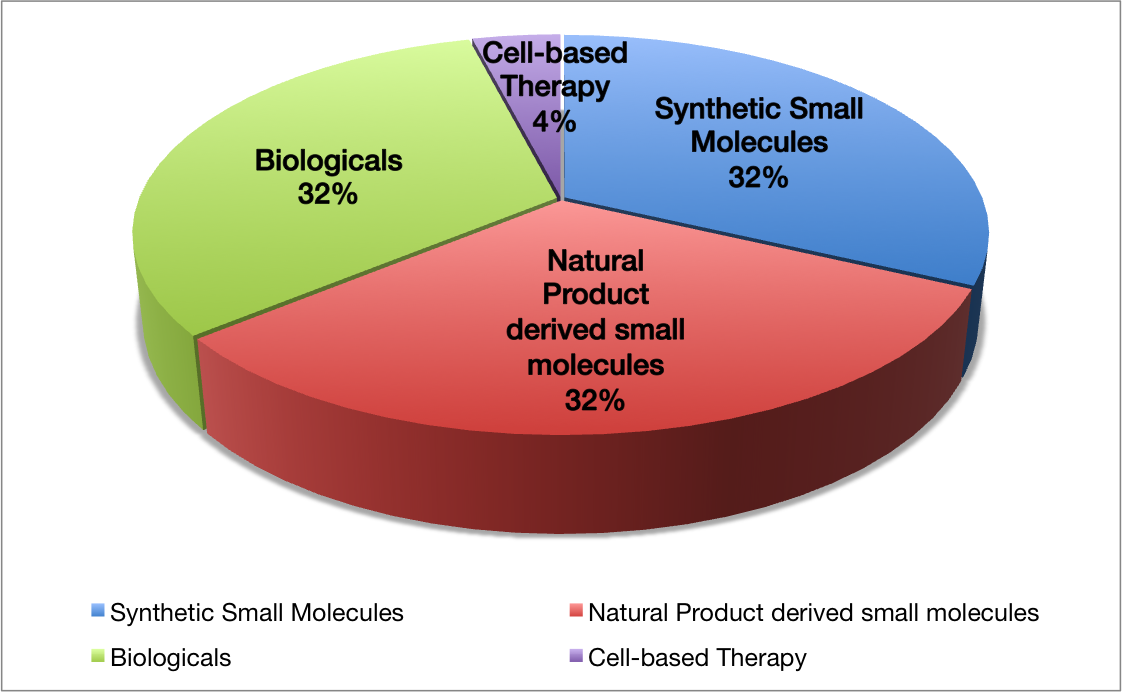
To overcome the obstacles connected with derivatisations in conventional synthesis and embrace the opportunity of substructure synthesis, nowadays synthetic chemists develop new strategic concepts of synthesis design. An example for such a novel concept is ANaPSyS (Artificial Natural Product Systems Synthesis).
ANaPSyS is based on structure pattern recognition and allows to synthesise biogenetically completely unrelated natural products based on their corporate molecular architecture by a shared synthetic sequence. This sequence is upgraded every time a synthesis makes use of it hence network diversification leads to a rise in revenue. Figure 2 contrasts our strategic approach to conventional synthesis. Thereby we take advantage of common structure motifs often shared by totally different natural products. It is this shared motif that we term “privileged intermediate (PI in red Figure 2)”, and which has to be identified and designed first. This is taken out by structural comparison and database searches. This intermediate is then synthesised, and diversified by means of synthetic chemistry into the respective natural products. For comparison, in conventional synthesis a target structure, which has been identified is synthesised via a specifically designed route. This route can only be used for the synthesis of this one individual molecule. Any changes made in the final structure require redesign and thus reinvestigation of the synthetic route (see Figure 2).
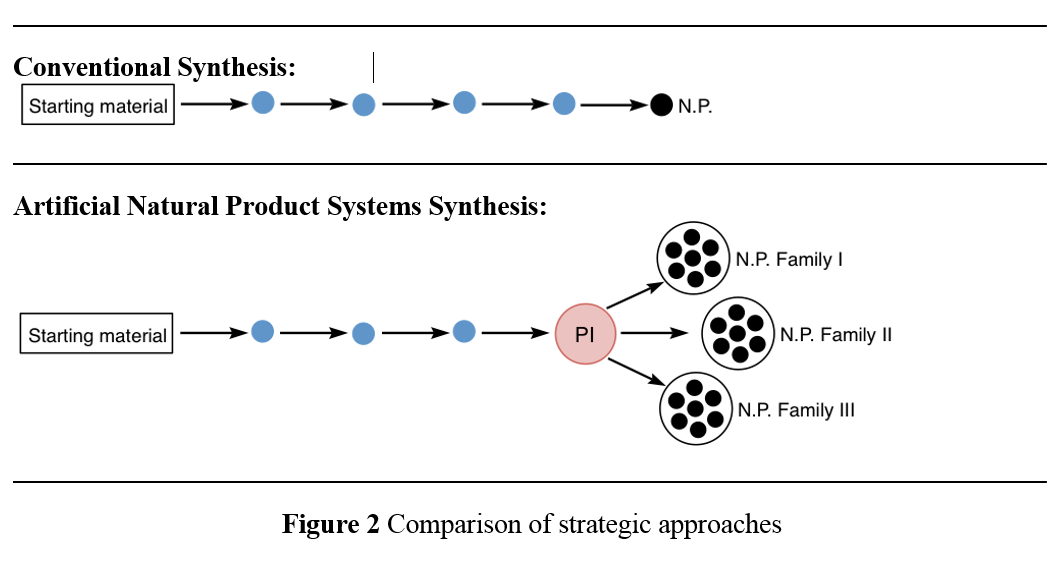
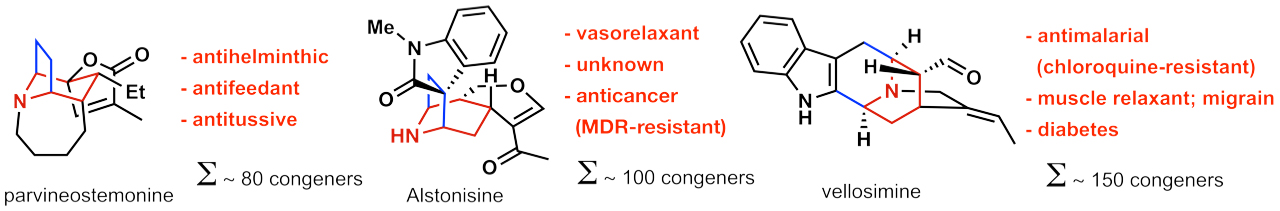
A specific example for such a research program is highlighted in Figure 3. Herein, 3 different natural product families with a broad range of pharmacological spectrum ranging from anti-cancer to anti-malarial activities (see Figure 4) share a common structure motif (highlighted in color in Figure 3). Up to date, the sum of chemical transformations required to synthesise 3 of these natural products (one congener per natural product family) is 69 steps. By contrast, ANaPSyS will only require 33 steps. The number of synthetic steps is thus cut in half, moreover the synthetic system created for these natural products is flexible and can be used to access any other molecule sharing the common structure motif.
This strategic approach thereby creates an artificial synthetic network suitable for efficient synthesis of natural products, their derivatives, and thus will provide access to their pharmacological properties as listed in figure 4. This will enhance efficacy in drug development, which is urgently required in pharmaceutical industry.
Prof. Dr. Tanja Gaich
University of Konstanz
Department of Chemistry
tanja.gaich@uni-konstanz.de
www.chemie.uni-konstanz.de/en/
Please note: this is a commercial profile