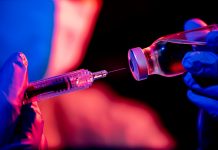
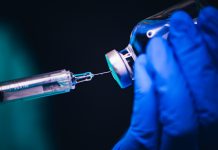
Today (20 November) Pfizer and BioNTech are sending their drug for approval to the FDA, meaning that the most vulnerable Americans could get the vaccine in December
The Pfizer and BioNTech vaccine was first announced to be 90% effective on 9 November, with promising data from their Phase Three clinical trials suggesting that the vaccine was close to being approved for use in the US.
Crucially, 42% of the participants were of diverse racial backgrounds, meaning that the vaccine will function to protect those who have demonstrated severe vulnerability to contracting and dying from COVID-19.
How close is the vaccine to being approved in the US?
CNN reported that the FDA will hold a meeting of its Vaccines and Related Biological Products Advisory Committee, which will occur over three days and then lead to a decision on 10 December about if Emergency Use Authorisation will be granted. Currently, the Pfizer and BioNTech collaboration is being filed for emergency use as the US Food and Drug Administration (FDA), which would fast-track the vaccine for public use as soon as mid-December. This pandemic is unprecedented and therefore fast, as this stage would normally take 10 months under the standard review process of the FDA.
Who could get the vaccine in December?
The NYTimes estimates that around 17 million to 20 million healthcare workers live in the US, with 1 million in nursing homes. This is the priority demographic for first use of the vaccine, which Pfizer officials said would produce roughly enough to immunise 20 million Americans before January. Other countries are still waiting to see what their regulatory authorities will decide, but it is likely that they will follow the same blueprint – nursing homes and healthcare workers first.
Have Pfizer and BioNTech filed for approval in other countries?
The companies have filed their vaccine with the EMA and the Medicines & Healthcare Products Regulatory Agency (MHRA) in the UK, and will submit applications to other regulatory agencies worldwide in the next few days. If Governments have a similar route to the FDA’s Emergency Use Authorisation, then officials at Pfizer state that they will be ready to “distribute the vaccine candidate within hours” of being cleared by regulatory authorities.
officials at Pfizer state that they will be ready to “distribute the vaccine candidate within hours”
Is the manufacturing and cold supply chain ready?
Pfizer officials say that they’re ready to combine their sites with BioNTech, creating the potential to supply up to 50 million vaccine doses globally in 2020. The figure for 2021 is up to 1.3 billion doses, which they say is highly depending on even more strong clinical trial result and how fast authorities can give them legal approval to start the process of manufacturing. Between them, the companies have sites in the US and Europe.
With regards to the cold chain, the intricate circular system of shipping and storage that keeps the vaccine at the right temperature, Pfizer has created a special temperature-controlled shipper for the vaccine. This can keep a temperature of -70C for up to 15 days, plus it will be tracked via a GPS-enabled thermal sensor to make sure each shipment is moving according to a strict international timeline. Once vaccines are thawed from their frozen cocoon, they can then be refrigerated at 2C-8C, for up to five days.
Dr Albert Bourla, Chairman and CEO at Pfizer, commented: “Filing in the U.S. represents a critical milestone in our journey to deliver a COVID-19 vaccine to the world and we now have a more complete picture of both the efficacy and safety profile of our vaccine, giving us confidence in its potential.”