Cancers resemble wounds. The question is why, and what does this mean? Many features of cancers – the so-called ‘Hallmarks of Cancer’ – may be mostly a wound-healing response. Dr Paul Edwards, Emeritus Reader at the University of Cambridge explores
Cancers behave like wounds, I suggest (1), because a cancer is disorganised tissue, and therefore it activates defence mechanisms that try and repair the disorganisation. These defence mechanisms are the same as those dealing with wounds: cancers are like wounds.
Cancer tissues share features with wounds
Cancer tissue is like wound tissue, as stated most clearly by Harold Dvorak in 1986. (2) Cancers are a mixture of cancer cells and normal cells embedded in a matrix, which is often like scar tissue and has new blood vessels. This ecosystem is called the ‘tumour microenvironment’. The normal cells include fibroblasts, which repair damage by making scar tissue; cells of the inflammation and immune systems, including lymphocytes and macrophages; and cells of the blood vessels that support them all. Apart from the cancer cells, this is the same as in a healing wound.
Recent work emphasises the similarity: MacCarthy-Morrogh and Martin (3) write that “The hallmarks of cancer [i.e. key characteristics, see below] are also the hallmarks of wound healing”. Researchers have, for example, identified ‘cancer-associated fibroblasts’, which are not like fibroblasts in fully normal tissue; but like the fibroblasts found in wounds. (4)
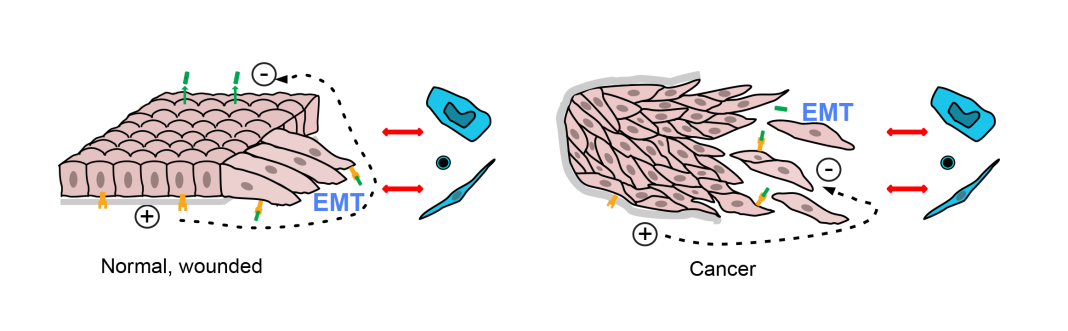
Similarly, shape changes, called the ‘epithelial-mesenchymal transition’ (EMT), are seen in epithelial cancer cells; but these shape changes are seen in wounded epithelial cells (3) as they migrate to close the wound. Even some of the lymphocytes present are not anti-cancer; they seem to be targeted to viruses that might invade a wound (see Ref. 1).
What is new – cause versus effect
How is this view different? Dvorak proposed that “tumors co-opted the wound-healing response to induce the stroma they required” (2); in other words, cancers use the facilities of wound-healing. The present proposal is that they inevitably trigger wound healing (they may, of course, also modify it, but this is another level of detail). Although this might seem an almost philosophical difference, it has important consequences.
Why is this important?
This is important because we need to distinguish between (a) cancer properties that are cancer-specific and that are the direct result of the mutations that cause cancer; and (b) downstream events that are the inevitable and normal consequence of tissue disorganisation. We need to distinguish between these if we are to understand how mutations convert normal cells into cancer cells; and to develop drugs that target cancer while sparing normal tissue. (As an aside, a recurring problem in cancer research has been to discover something interesting in cancers and to assume it is cancer-specific, only later to realise it is normal biology). (1)
There is now a case for re-examining the ‘Hallmarks of Cancer’. These are a tentative list of the properties of cancer tissue, suggested by Hanahan and Weinberg (5), but many of the ‘Hallmarks’ are also hallmarks of healing wounds. (3) We should now separate hallmarks that are cancer-specific from those – such as ‘EMT’ and angiogenesis –that are normal, built-in reactions to tissue damage. The typical mutations of common cancers seem mainly to alter differentiation, proliferation and survival, or genetic instability (consider, for example, the typical mutations found in colon cancer). (6) What is more, turning on a single growth-control gene, MYC, can turn a benign growth into a malignant cancer complete with the features of wound-healing, while the tissue structure of cancers seems to be “principally specified by their host tissue”. (7)
Reinterpretation, not rejection
This view doesn’t invalidate current observations, but may make more sense of them. Much current cancer research studies the interactions between the cancer cells and the normal neighbouring cells that make up the tumour microenvironment. For example, apparently, lymphocytes are encouraged to attack cancers by the amino acid aspartate. Why? Perhaps release of aspartate from damaged cells is a damage signal. And the many recent observations of how immune responses to cancers are modulated may reflect complex regulation of inflammation and immunity in wounds.
Some disorganisation signals are known
We know a little – but only a little – about the alarm signals that disorganised or damaged tissue sends out. (1) For epithelia (the surface sheets of the body – skin, gut, lung, breast ducts, prostate gland, etc, that give rise to the commoner adult cancers), we know that they are normally a continuous sheet with a distinct inside and an outside, separated from underlying tissue by the basement membrane (see Diagram). There is a salt difference and an electrical potential across the sheet.
A break in the sheet causes electric currents, and allows signal molecules that are normally confined to one side to reach receptors (i.e. detectors) on the other side. Both seem to be important signals – for example, the currents direct migration of cells over wounds. Doubtless, neighbouring non-cancer cells respond and also likely react to inappropriate contacts where the basement membrane is breached.
Conclusions
One conclusion is that we ought to find out more about how tissues sense, and correct, disorganisation of their structure. We know some signals of disorganisation of epithelia, but there must be many other such mechanisms, notably those for other cell types. This seems to be an under-researched area.
Another is the need to clarify which features of cancers are truly unique to cancers, either because they are the direct consequences of gene changes that drive cancer development or because they are not simply normal responses to tissue disorganisation.
References
- Edwards PAW (2023) J Pathol 260,1-4.
- Dvorak HF (2015) Cancer Immunol Res 3, 1–11.
- MacCarthy-Morrogh L & Martin P (2020) Sci. Signal. 13, eaay8690
- Biffi G & Tuveson DA (2021) Physiol Rev 101, 147.
- doi: https://doi.org/10.1152/physrev.00048.2019
- Hanahan D & Weinberg RA (2011) Cell 144, 646–674
- Jones S et al (2008) Proc Natl Acad Sci USA 105, 4283–4288
- Sodir NM et al (2020) Cancer Discov 10, 588.