Drawing on his research in understanding factors impacting infection tolerance, Brian P. Lazzaro, Liberty Hyde Bailey Professor at Cornell University, discusses the importance of tolerance to minor infections, highlighting that while active immune responses are crucial for pathogen defense, tolerance can often lead to better health outcomes
Active immune responses that kill pathogens dominate our thinking about defense against infection. However, tolerance of low-grade infection is equally important and, in many cases, provides the path to the greatest health. Strategies or interventions to increase tolerance could be instrumental in promoting public health, but our current understanding of the mechanisms and consequences of tolerance is insufficient for the development of effective strategies.
The concept of tolerance originates in agriculture, describing crop strains that are able to deliver high yields while sustaining pathogen burdens that would decimate standard cultivars. This is differentiated from resistance, which is the ability to suppress infection through immune responses that kill pathogens. From a grower’s perspective, strains that produce high yields even while infected are just as valuable as those that are resistant to infection. Analogously, sustaining health by tolerating minor infections is just as good as – and in some cases, better than – trying to eradicate the infection with an intense immune response or aggressive therapeutics.
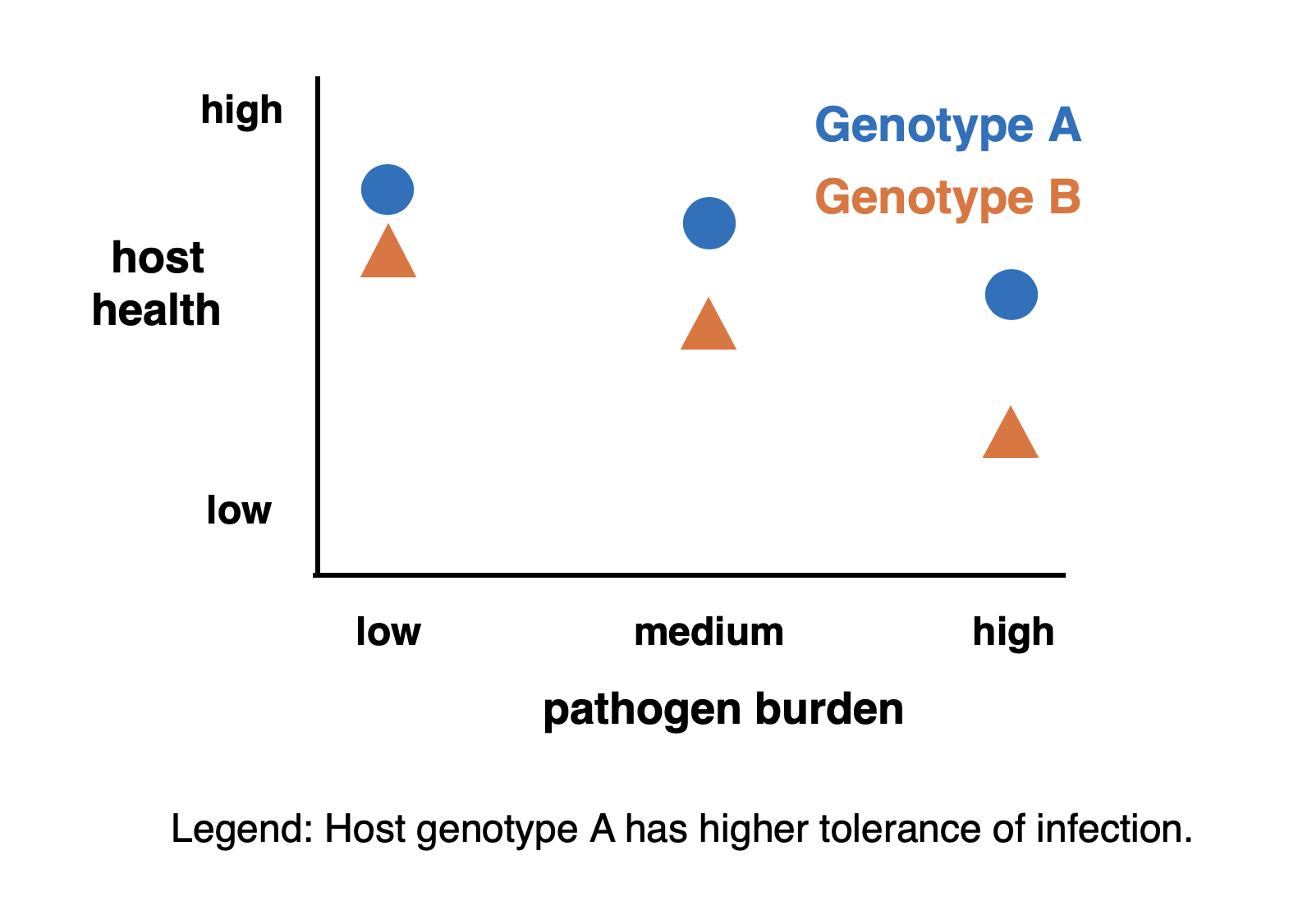
How do we measure tolerance?
The concept of tolerance may be intuitive, but measuring it can be a challenge. In principle, we want to measure the extent to which health is sustained despite infection. We naturally expect the severity of health consequences to scale with the severity of the infection, so we need to evaluate tolerance across individuals experiencing a range of infection intensities. We measure tolerance as the magnitude of retained health, scaled against pathogen burden. In the illustrative figure, individuals with Genotype A are said to have a higher tolerance of infection than individuals with Genotype B because they retain more health at each burden, even though health declines with increasing pathogen load for both host genotypes. In experimental settings, we can longitudinally measure health before and after experimental infections while carefully controlling host genotype and environmental conditions. However, generating and interpreting data becomes more difficult when the measurements are performed on humans or other organisms living in natural populations, as infection severity can be conflated with genetic or environmental differences among the individuals, prior exposure histories, and other uncontrolled variables.
Very often, we are specifically interested in how environmental or genetic factors might contribute to tolerance of infection. Using laboratory model organisms such as the fruit fly Drosophila melanogaster, we can measure tolerance in genetically identical individuals provided with different environmental conditions. We can also measure differences in infection tolerance of genetically distinct individuals provided with shared environmental conditions. In one such study, our research group found that genetic diversity and dietary nutrition combine to determine tolerance of, as well as resistance to, bacterial infection in D. melanogaster.
Nevertheless, even under controlled conditions, experimental design and interpretation of results can be nuanced. Host health metrics could include physiological traits, reproductive success, crop yield, or even probability of surviving infection. But how do we ensure that summary metrics accurately describe overall health? Pathogen burden is typically measured as the number of parasites in the body. But when should pathogen burden be measured if it is assumed to predict host health? Should it be recorded at the same time that the health metric is measured? Twenty-four hours earlier? A week? Furthermore, while pathogen burden is certainly an important infection trait, a narrow focus on absolute pathogen number disregards potential behavioral or physiological differences between the pathogens in different hosts. This is especially important because pathogen behavior and virulence can change as a function of pathogen density. There is a pressing need to develop more sophisticated statistical models that capture tolerance by integrating multiple host metrics in non-linear functions while accounting for distinct pathogen behaviors at varying burdens.
Mechanisms of tolerance
One hindrance to the study of tolerance is the black-box nature of the phenomenon. Whereas core elements of the immune system can be readily defined, the potential mechanisms through which tolerance may arise are considerably more varied. Intuitive tolerance mechanisms include repair of pathogen-induced damage and detoxification of pathogen-derived virulence factors. Such mechanisms limit the consequence of infection without directly targeting the pathogen itself. Tolerance can also emerge from metabolic homeostasis, which enables the host to withstand an infection until it is resolved by the immune system. Crucially, potential tolerance can be determined by developmental and environmental variables that shape the overall host condition long before infection occurs. For example, past nutritional conditions can enable future tolerance of infection by building energetic stores. The broad physiological base of tolerance makes it harder to identify dedicated tolerance mechanisms but provides many more avenues for intervention and bolstering capacity for tolerance.
One strategy to elucidate tolerance mechanisms is to map the genes contributing to variation in the ability to tolerate infection. In one such project, we performed a genome-wide association study to map the genomic basis for variation in tolerance of bacterial infection by D. melanogaster. We found that many genes in physiologically distinct processes make small contributions to overall variation in tolerance, including genes that regulate damage repair and metabolism. This differs from the genetic architecture of resistance, where variation in a small number of immunological genes can have a large impact on relative resistance or susceptibility to infection. Comparative genetic mapping across biological systems will reveal commonalities and differences in tolerance-determining mechanisms, which can then enable the development of strategies to promote tolerance in managed settings.
How separate are resistance and tolerance?
While they are conceptually distinct, resistance and tolerance are often interwoven in reality. Our genetic mapping study found that tolerance was strongly positively correlated with resistance. While this is a common pattern, other studies have also found resistance and tolerance to be negatively correlated, or even unrelated to each other.
There are plausible reasons to expect resistance and tolerance to be correlated. While tolerance mechanisms that repair damage or neutralize toxins may have no direct effect on the pathogen, there may be indirect consequences if virulence promotes pathogen success. When a pathogen uses a toxin to exploit a host resource, neutralization of that toxin prevents the pathogen from accessing the resource, thereby indirectly inhibiting the pathogen. Thus, what would appear to be a tolerance mechanism simultaneously increases resistance. The same is true for energetic reserves that support the immune system while also maintaining general body functions. Any deficiency in those reserves constrains the immune system, reducing resistance to infection, and, in parallel, impairs homeostasis and decreases infection tolerance. Alternatively, resistance and tolerance can be negatively correlated when the immune response is itself damaging. Overactive immune responses may be extremely effective at eliminating infections, but the collateral autoimmune damage manifests as reduced tolerance.
The physiological demands of resistance can lead to a collapse in tolerance, particularly in individuals who are already stressed. We examined defense against bacterial infection in D. melanogaster females who were reproductively active in comparison to those who were not. We found that the reproductively invested females were much more likely to die from their infections, and that the sensitivity arose from a joint failure of resistance and tolerance. In this example, the consequence emerges from the shared use of a polyfunctional tissue for reproductive provisioning, the immune system, and metabolic control.
In insects, the fat body is the primary systemic immune organ and is also the tissue that produces egg yolk proteins. Furthermore, as the name suggests, and in analogy to the mammalian liver, the fat body stores lipids and regulates metabolism. We found that when reproductively active D. melanogaster were given a bacterial infection, the combined demand of yolk production and immune system activity overwhelmed the fat body. The tissue’s capacity to synthesize new proteins declined, impairing the immune response and decreasing resistance while also undermining infection tolerance. The simultaneous failure of resistance and tolerance resulted in dramatically higher probability of host death.
The resistance versus tolerance dichotomy has been valuable for expanding our conception of defense against infection, but integrating the two will become essential moving forward. Efforts to increase health by promoting tolerance will need to account for tissues and mechanisms that are shared between resistance and tolerance and for feedback between them.
How does tolerance impact host-pathogen co-evolution?
There is a well-developed body of scientific literature on how immunological resistance drives pathogen evolution, including escalating ‘co-evolutionary arms races’ in pathogen virulence and host immunity. In contrast, scientific theory on how pathogens are expected to evolve in response to host tolerance is considerably less well developed.
Foundational mathematical models describing host tolerance have suggested that host-evolved tolerance should dampen pathogen evolution of virulence. This prediction is based on the core assumptions that virulence mechanisms are costly for the pathogen to produce and unnecessary in a tolerant host. In these models, the evolution of increased tolerance by the host enables pathogens to dispense with costly virulence mechanisms, de-escalating host-pathogen antagonism to benefit both parties. Taken to a logical extreme, evolved host tolerance could facilitate the evolution from pathogenic relationships into benign commensalisms or even beneficial mutualisms. Indeed, host tolerance is a prerequisite for host- microbe mutualism. Evidence for coevolutionary de-escalation can be found in the observation that many mutualistic bacterial symbionts are evolutionarily derived from pathogens. However, there are also reasons to be sceptical of tolerance-based de- escalation models.
There are multiple reasons why host tolerance might not universally promote de-escalation of virulence. For one, pathogen virulence is not always directed against the host. Virulence can sometimes be a side effect of competition between pathogen strains co-infecting a single host. For example, faster-growing parasites may have a competitive advantage over slower-growing strains in a co-infected host, with pathogenic consequences to the host emerging as a collateral side effect. In this case, host tolerance of the parasite does not affect the competition between parasite strains and may even create space for enhanced competition, leading to increased virulence. Additionally, virulence is sometimes an obligatory component of pathogen transmission. Natural selection will never drive the evolution of reduced virulence if it comes at the cost of reduced transmission.
The host may also be constrained in the degree of tolerance that it can evolve. Classical genetic models of tolerance evolution suggest that alleles that increase tolerance are adaptively favorable because they alleviate costs in both host and pathogen. Clearly, though, there must be limits. The host cannot be infinitely tolerant of infection; at some point, the metabolic cost of sustaining a large pathogen population would prohibit the evolution of further increases in tolerance. For this reason, we don’t see plants and animals dispensing with their immune systems entirely and investing fully in strategies of peaceful accommodation. Even organisms with strong mutualistic relationships with bacteria or fungi use their immune systems to regulate symbiont number, lest the symbiont become a pathogen through overproliferation.
Our research team and others have observed considerable genetic variation among individuals in natural populations in their capacity for infection tolerance. These observations are inconsistent with the hypothesis that tolerance is always adaptively favorable. Instead, we have suggested that genetic variation for tolerance is likely to be maintained in natural populations through the correlation between resistance and tolerance, environmental heterogeneity, and evolutionary costs of tolerance that are dependent on overall host condition. New theory that integrates these empirical observations must be developed to create a more realistic understanding of how resistance and tolerance are balanced in maintaining health during infection.
Conclusion and outlook
All plants and animals – including ourselves – are constantly exposed to the environment’s microbes, parasites, and pathogens. While we rely on our immune systems to protect us, it would be impossible (and undesirable) to immunologically or therapeutically eliminate every foreign organism we encounter. Instead, we depend on tolerance mechanisms for maintaining health despite minor infections. Enhancing tolerance presents a promising strategy for increasing public health and agricultural productivity, but our fundamental understanding of the mechanisms and consequences of tolerance is currently insufficient. Ongoing comparative studies by our research team and others are defining these mechanisms and building the mathematical theory for an optimized balance between resistance and tolerance of infection.