FDA clearance of the first Alzheimer’s blood test marks a significant step toward earlier, more straightforward diagnosis, potentially improving care for adults with memory concerns
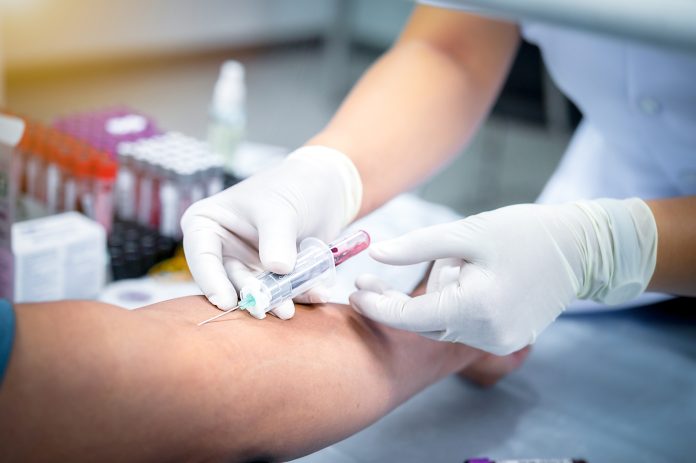
In a significant step forward for dementia care, the U.S. Food and Drug Administration (FDA) has approved the first blood test to aid in diagnosing Alzheimer’s disease. The test, which offers a simpler, less invasive alternative to brain scans and spinal taps, has the potential to transform how and when the condition is identified, especially for patients showing early symptoms. This accessibility and simplicity bring a sense of optimism and empowerment to healthcare professionals and patients alike.
Lumipulse blood test: A significant milestone for Alzheimer’s disease
Alzheimer’s disease is a progressive brain disorder and the most common cause of dementia. It leads to memory loss, confusion, changes in behavior, and difficulties with thinking, speaking, and performing daily tasks. It typically affects people over age 65, but can begin earlier.
The Lumipulse blood test represents a significant advancement in the understanding and diagnosis of Alzheimer’s disease. This innovative test quantitatively evaluates the ratio of two critical proteins: pTau217 and ß-Amyloid 1-42, both of which play vital roles in the development and progression of Alzheimer’s. Remarkably, this test offers a non-invasive and cost-effective alternative to traditional diagnostic methods, eliminating the need for complex and expensive procedures while providing valuable insights into the disease’s presence.
“This blood test offers a simpler, less invasive, and more widely accessible way to support accurate detection of amyloid plaques and tau tangles, two of the main pathological markers of Alzheimer’s disease,“ Michal Schnaider Beeri, director of the Herbert and Jacqueline Krieger Klein Alzheimer’s Research Center within the Rutgers Brain Health Institute, commented.
In clinical studies, the blood test showed exceptional results, with 91.7% of individuals who tested positive having amyloid plaques confirmed through PET scans or cerebrospinal fluid tests, and 97.3% of those with negative results showing no signs of pathology. The test’s accuracy surpasses many conventional cognitive assessments and brings accessible diagnostics to the millions of individuals living with Alzheimer’s.
“With approximately 90% accuracy, this blood test enables clinicians to identify Alzheimer’s disease, once initial symptoms have become noticeable,“ said Schnaider Beeri, adding that this can significantly improve outcomes and quality of life for patients and families, offering hope and reassurance where barriers once stood.
However, Schnaider Beeri cautioned that the new test has limitations. She said. “It does not reliably predict future dementia in people without symptoms, limiting its use for prevention, and it focuses only on amyloid plaques and tau tangles, while Alzheimer’s and related dementias involve other factors like vascular disease and neuroinflammation.”
Closing diagnostic gaps
The Krieger Klein Alzheimer’s Research Center in the Rutgers Brain Health Institute is dedicated to closing diagnostic gaps through pioneering research on metabolic factors, digital cognitive tools, and other biological pathways linked to neurodegeneration. This dedication instills confidence and a sense of support in healthcare professionals and patients.
“Our research is dedicated to advancing early detection and intervention strategies for Alzheimer’s disease,“ Schnaider Beeri said. “The introduction of this blood test aligns closely with our ongoing efforts to integrate new biomarkers into our cohort studies and clinical trials. It enhances our ability to identify at-risk individuals and tailor interventions more effectively.”