The Canadian Rare Disease Network (CRDN) is uniting care, research, and lived experience to improve the rare disease journey in Canada
Over 3 million people across Canada are affected by 7,000+ known rare diseases (RDs), many of which are severe, progressive, and life-limiting [1, 2]. These numbers reflect real people: children spending their early years in hospitals, parents turned full-time caregivers and advocates, and adults facing daily uncertainty about their health and future. Yet patients continue to fall through cracks of Canada’s health and research systems because they don’t fit the mold.
The Canadian Rare Disease Network (CRDN)/ Réseau Canadien des Maladies Rares (RCMR) was launched to change this reality – connecting people, priorities, and projects across the country to close those gaps, accelerate progress, and ensure that no patient or family is left behind because their condition is rare.
Canada’s system does not work for rare
Canada’s systems are designed for common, well-understood conditions with clear clinical pathways, established treatments, and large patient populations. RDs, by their very nature, defy these norms. For instance, one Canadian teen faced congenital heart defects, hearing loss, and skeletal abnormalities – yet was not referred to genetics until age 17. After 150+ medical appointments and years without answers, an AI-powered tool finally prompted the referral and diagnosis. Stories like this are far from unique. Across the country, people with rare or atypical conditions face long diagnostic delays – averaging 3.7 years [3]. Access to treatments, when available, is uneven, and psychosocial supports are fragmented. Too often, families are left to become their own care coordinators, advocates, and navigators through a maze of disconnected services.
Canada’s structural realities deepen these cracks. With a relatively small population and limited healthcare professionals spread across the world’s second-largest country, access to specialized care is uneven – particularly when RD expertise is concentrated in few urban locations. On top of this, Canada has a patchwork of 13 publicly funded provincial and territorial health systems, with their own policies, priorities, and capacity. This fragmentation results in significant variation in access, testing, and follow-up care.
A national turning point
For decades, patients and advocates have called for coordinated national action. In March 2023, that call was partly answered with the launch of Canada’s National Strategy for Drugs for Rare Diseases, backed by a $1.5 billion federal investment and the creation of a RD Directorate within Health Canada [4]. This landmark investment signalled historic recognition of RDs as a national priority.
The momentum is timely. Advances in genomics, AI-powered diagnostics, precision medicine, and “N-of-1” treatments are transforming possibilities. Canada has the expertise – world-class researchers, clinicians, and patient leaders. What has been missing is an integrated network to bring these people and innovations together.
CRDN: Connecting the pieces
CRDN was launched in 2024 to fill this gap. With initial support from One Child Every Child, CRDN is a pan-Canadian, cross-sector initiative designed to activate, connect, and coordinate key actors to accelerate progress. Its power lies in the community it connects.
More than a research consortium or advocacy organization, CRDN is the connective tissue that links healthcare, research, and patient communities around a shared vision: Innovative care and research so all patients and families affected by a RD are empowered to live their full potential.
That vision is about more than medical breakthroughs – it is about integrating research and care so that discoveries quickly translate into practice, and care experiences inform the next generation of research. It is about recognizing that improving quality of life for people with RDs means addressing the full spectrum of need – medical, psychological, financial, social, and educational.
Umbrella patient organizations like the Canadian Organization for Rare Disorders (CORD) and Regroupement Québécois des maladies rares (RQMO) are central to this work, ensuring that community needs and lived experience drive collective action.
“We often say that CRDN is not building something new from scratch – we’re building on what’s already there and making it stronger by connecting everything together,” says Ian Stedman, a lawyer, professor, advocate, and CRDN Steering Committee member living with a RD.
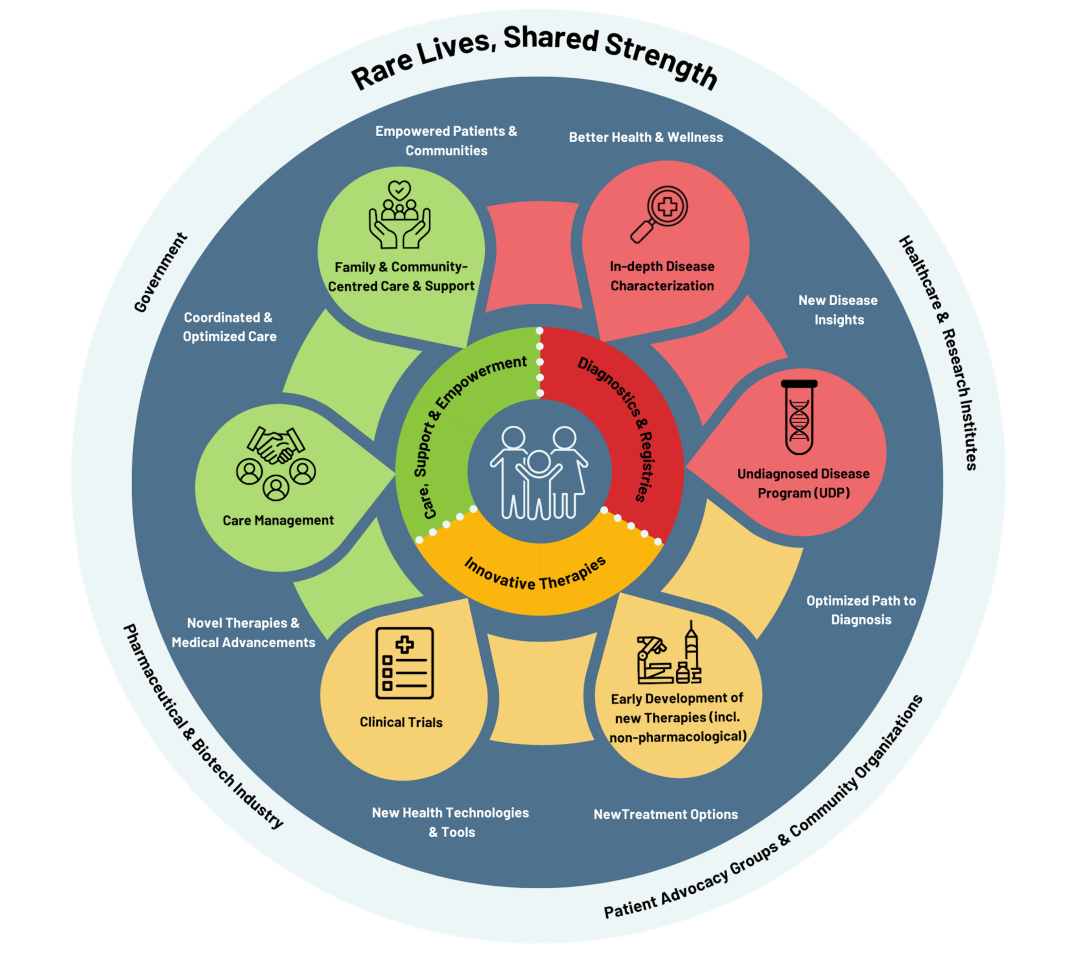
CRDN’s strategic framework, developed by and for Canada’s RD community, is organized around four interrelated pillars, each focused on improving the RD landscape:
- Diagnostics and registries (Led by Dr. Kym M. Boycott): Improving equitable access to timely, accurate diagnosis, harmonized newborn screening, and interoperable data systems and registries.
- Innovative therapies (Led by Dr. Leanne M. Ward): Strengthening Canada’s ability to design, test, and deliver promising therapies – pharmacological and non-pharmacological – across the full pipeline from discovery to access.
- Care, support, and empowerment (Led by Dr. Ian Stedman): Addressing the broader needs of people with RDs, including care navigation, psychosocial support, and community-based services
- National and global collaboration: Aligning RD efforts across Canadian provinces, organizations, and sectors, and connecting with global RD initiatives to both contribute and learn from.
Each pillar is designed with a dual lens: leverage and strengthen what already exists, while identifying and filling gaps with forward-looking systems. This ensures the framework is both pragmatic and aspirational, grounded in current realities, while still ambitious enough to drive transformative change.
From cracks to connections
CRDN’s ultimate aim is to help Canada evolve into a learning health system for RDs, where care and research are not parallel tracks but integrated pathways with patients as active partners. In this system, data, evidence, and lived experience continuously shape policies, practices, and innovation.
By connecting people, priorities, and infrastructure – and reshaping systems to serve those who don’t fit the mold – Canada can build a future where RD patients and families are fully supported to live their best lives.
References
- Haendel, M., et al., How many rare diseases are there?
Nat Rev Drug Discov, 2020. 19(2): p. 77-78. - Ferreira, C.R., The burden of rare diseases. Am J Med
Genet A, 2019. 179(6): p. 885-892. - Canadian Organization for Rare Disorders, Rare Disease Survey 2023: Experiences of Rare Disease Patients. 2023.
- Health Canada. The National Strategy for Drugs for Rare Diseases. March 22, 2023; Available from: https://www.canada.ca/en/health-canada/services/health-care-systems/national-pharmacare/strategy-drugs-rare-diseases.html