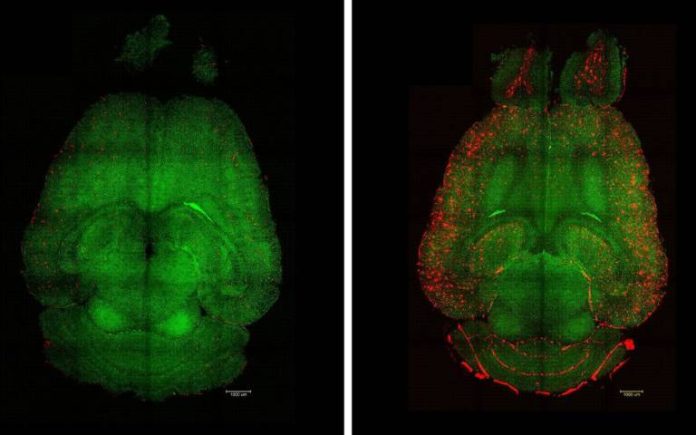
A UCL-led study shows bioactive nanoparticles can restore blood-brain barrier function and clear amyloid-β in mice, reversing Alzheimer’s-like brain changes
A new UCL-co-led study reveals that specially designed nanoparticles can reverse Alzheimer’s disease pathology in mice by repairing the blood-brain barrier and promoting the removal of toxic amyloid-β proteins. Unlike traditional approaches that deliver drugs, these “supramolecular” nanoparticles act as therapeutic agents themselves. In treated mice, scientists observed dramatic reductions in amyloid burden and even restoration of normal behaviour over time. This discovery opens a promising pathway toward therapies that target brain vasculature to combat dementia.
The findings are detailed in Signal Transduction and Targeted Therapy.
Repairing the brain’s vascular system to clear harmful proteins
Unlike traditional nanomedicine, which uses nanoparticles to deliver drugs, this approach employs bioactive nanoparticles that act as treatments themselves. Rather than targeting neurons, the therapy focuses on repairing the blood-brain barrier—the brain’s protective gateway. Restoring this crucial interface allowed researchers to reverse Alzheimer’s-related damage in animal models, offering a potential new route to treating dementia.
The brain obtains energy from a vast blood supply, supported by a unique and dense vascular system, where a single capillary nourishes each neuron. The brain contains around one billion capillaries, highlighting the vital role of brain vasculature health, especially in diseases like dementia and Alzheimer’s.
The blood-brain barrier is a cellular and physiological barrier that separates the brain from the blood flow, protecting it from external dangers such as pathogens or toxins.
The supramolecular nanoparticles developed in this study act like a switch that resets the system. It mimics the ligands of LRP1, effectively binding to amyloid-β, crossing the blood-brain barrier, and initiating the process of removing harmful proteins from the brain. This helps restore the vasculature’s natural role as a waste-clearing pathway and bring it back to proper function.
Introducing nanoparticles to treat Alzheimer’s disease
The nanoparticles act as supramolecular drugs and are designed with a bottom-up molecular engineering approach, allowing precise size control with a defined number of surface ligands. The platform is then able to interact with multiple cellular receptors in a particular way, thereby opening up a unique and novel way to regulate receptor function.
This precision enables the adequate clearance of amyloid-β from the brain and restores balance to the vascular system that maintains healthy brain function.
Professor Lorena Ruiz Perez, based at IBEC and the University of Barcelona (UB), said: “Our study demonstrated remarkable efficacy in achieving rapid Aβ clearance, restoring healthy function in the blood-brain barrier and leading to a striking reversal of Alzheimer’s pathology.”
Removing waste proteins from the brain and restoring health
The researchers found that targeting a specific mechanism allowed undesirable “waste proteins” produced in the brain to pass through this barrier and be eliminated in the blood flow. In Alzheimer’s disease, the main “waste” protein is amyloid beta.
Researchers used genetically programmed mice that developed a significant cognitive decline mimicking Alzheimer’s pathology. They administered only three doses of the supramolecular drugs, and the mice were regularly monitored.
Dr Junyang Chen, first co-author of the study based at the West China Hospital of Sichuan University, who was a PhD student at UCL Chemistry during the work, said: “Only one hour after the injection, we observed a reduction of 50–60% in Aβ amount inside the brain.”
The researchers found that one 12-month-old mouse (equivalent to a 60-year-old human), who was treated with the nanoparticles, had recovered to a healthy mouse six months later.
Study leader Professor Giuseppe Battaglia, formerly a professor at UCL Chemistry and now a UCL visiting professor, based at the Catalan Institution for Research and Advanced Studies (ICREA) and the Institute for Bioengineering of Catalonia (IBEC), said: “The long-term effect comes from restoring the brain’s vasculature. We believe it works like a cascade: when toxic species, such as amyloid-beta (Aβ), accumulate, the disease progresses. However, once the vasculature can function again, it begins to clear Aβ and other harmful molecules, allowing the entire system to regain its balance.
“What’s remarkable is that our nanoparticles act as a drug and seem to activate a feedback mechanism that brings this clearance pathway back to normal levels.”