In this article, Prof Dr-Ing. Jörg Volpp from University West, Trollhättan in Sweden, explores how surface tension in pure metals behaves at high temperatures, particularly in the boiling range
When increasing the temperature of a pure metal from its melting temperature, its surface tension decreases. Often, a linear decrease is assumed (e.g., (1)). However, it is likely that surface tension does not disappear at a high temperature. For example, within a laser-generated vapor channel, the temperature increases to levels above the boiling point, yet surface tension remains active. (2) A steeper decrease in the surface tension coefficient was observed with stagnation just below the boiling temperature, (3) whereas above the boiling temperature, an increase was seen. (2) This surprising effect can have different origins. One possible effect is the impact of ejecting atoms, which leave holes in the surface and thereby change the bonding forces within the surface, a measure of surface tension.
Surface tension
Surface tension plays a crucial role in various high-temperature processes, including welding, brazing, cutting, and additive manufacturing, among others. It often defines the shape and geometry of the resulting tracks or edges as a result of the operation. However, during the processing, when the material is still liquid, surface tension impacts the melt flow behavior and dynamics. One important effect is the Marangoni flow induced by surface tension differences. Surface tension is defined as the mechanical force parallel to the surface. Attractive bonding forces between the surface atoms define the surface tension coefficient value. In general, as the temperature increases, the bonding forces decrease, and the atoms require more space due to increased vibration, resulting in thermal expansion and reduced surface tension.
Boiling metal surface
When the temperature increases, more surface atoms leave the metal surface. At extensive boiling, the ejections outnumber those of the atoms condensing, and a non-equilibrium state is induced. The atoms leave the surface at high speeds, and a thin so-called Knudsen layer appears, in which the atoms accelerate and create a recoil pressure on the surface. However, ejecting atoms leave a hole in the surface, which in turn leads to altered conditions regarding bonding forces within the surface layer and, therefore, also changes in surface tension. It is not fully clear how the atoms eject and what effect the hole patterns have on the surface tension.
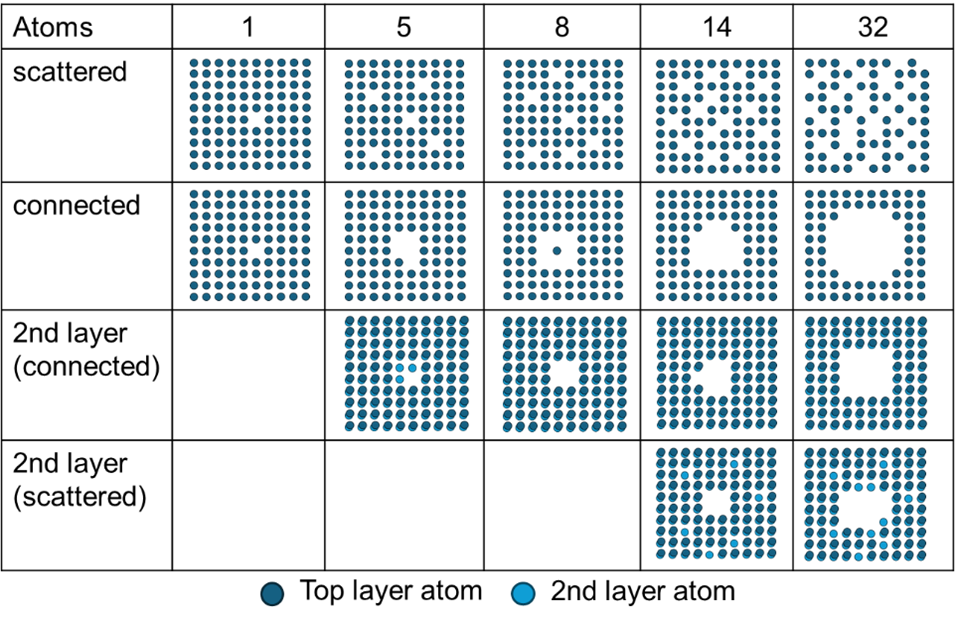
Therefore, a theoretical model was developed to simulate a cube of 10x10x10 iron atoms and calculate the surface tension at different temperatures. (4) This model was modified to consider atom removals from the surface and derive surface tension coefficients based on the new surface structure. The vaporization rate was calculated based on different bulk temperatures. Different patterns were assumed to be removed (Fig. 1). Scattered removal assumed that single atoms at different positions eject, while connected patterns assumed crater creation. In addition, patterns were also defined that affect the second atom layer.
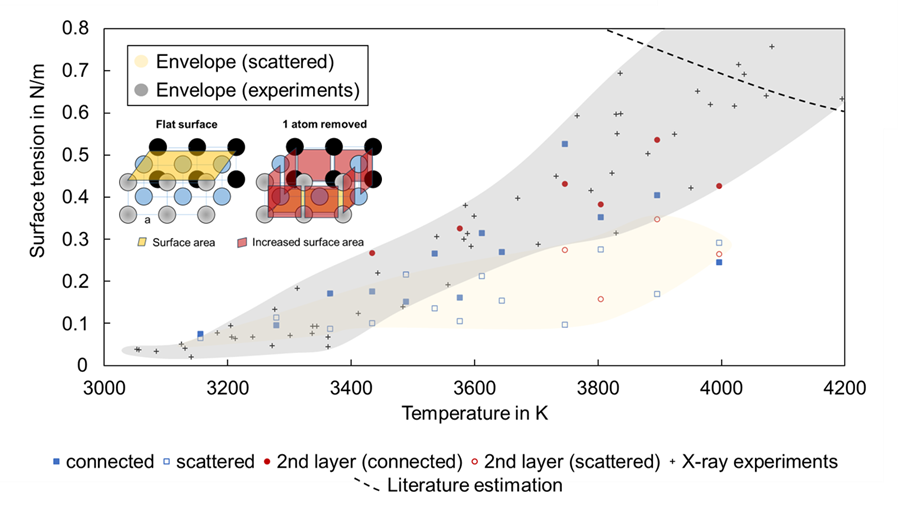
The calculated surface tension coefficients show a similar trend compared to previous measurements (Fig. 2). (3) An increasing trend was observed. The connected patterns follow the measured values quite well, while the scattered patterns tend to underestimate the surface tension coefficient values.
Those observations suggest that the removal pattern at high temperatures and the related ejection rates form a connected pattern. Such patterns appear to induce surfaces and surface forces that increase surface tension as temperatures rise.
Additionally, it can be observed that scattered patterns result in lower surface tension values. This helps to explain the observed steep decrease in surface tension (2) below the boiling point.
In summary, the ejection pattern of the atoms defines whether surface tension is increased or decreased. The newly created knowledge can help to develop more energy-efficient material processing techniques to reduce the high energy needs of the manufacturing industry.
Acknowledgements
Vetenskapsrådet (The Swedish Research Council), SMART – Surface tension of Metals Above vapoRization Temperature (2020–04250)
References
- Keene, B. J. (1993). Review of data for the surface tension of pure metals. International Materials Reviews, 38 (4), 157-192.
- Volpp, J., Sato, Y., Tsukamoto, M., Rathmann, L., Möller, M., Clark, S. J., Fezzaa, K., Radel, T., Klingbeil, K. (2024). The surface tension of boiling steel surfaces. Results in Materials, 100583.
- Volpp, J. (2023). Surface tension of steel at high temperatures. SN Applied Sciences, 5(9), 237.
- Volpp, J. (2023). Laser light absorption of high-temperature metal surfaces. Heliyon, 9(10).