Jolt has developed novel organic batteries that could foster the widespread adoption of renewable energy sources for electricity generation
The global appetite for electrical energy is rapidly growing, fueled partly by increased demand for electric vehicles, artificial intelligence, and cryptocurrency. Global electricity use is predicted to exceed 32,000 terawatt-hours (TWh) by 2026 (Electricity 2024, International Energy Agency). At the same time, concerns about climate change have driven a focus toward low-emission sources. Indeed, electric power generation from renewables will likely outpace total demand growth, at least in the US and Europe. However, while the cost of energy production via wind and solar has decreased significantly in recent years, the intermittent nature of these sources requires an efficient, long-duration (> 8 hrs) energy-storage technology, significantly adding to the total project cost.
The search for sustainable grid-level storage solutions
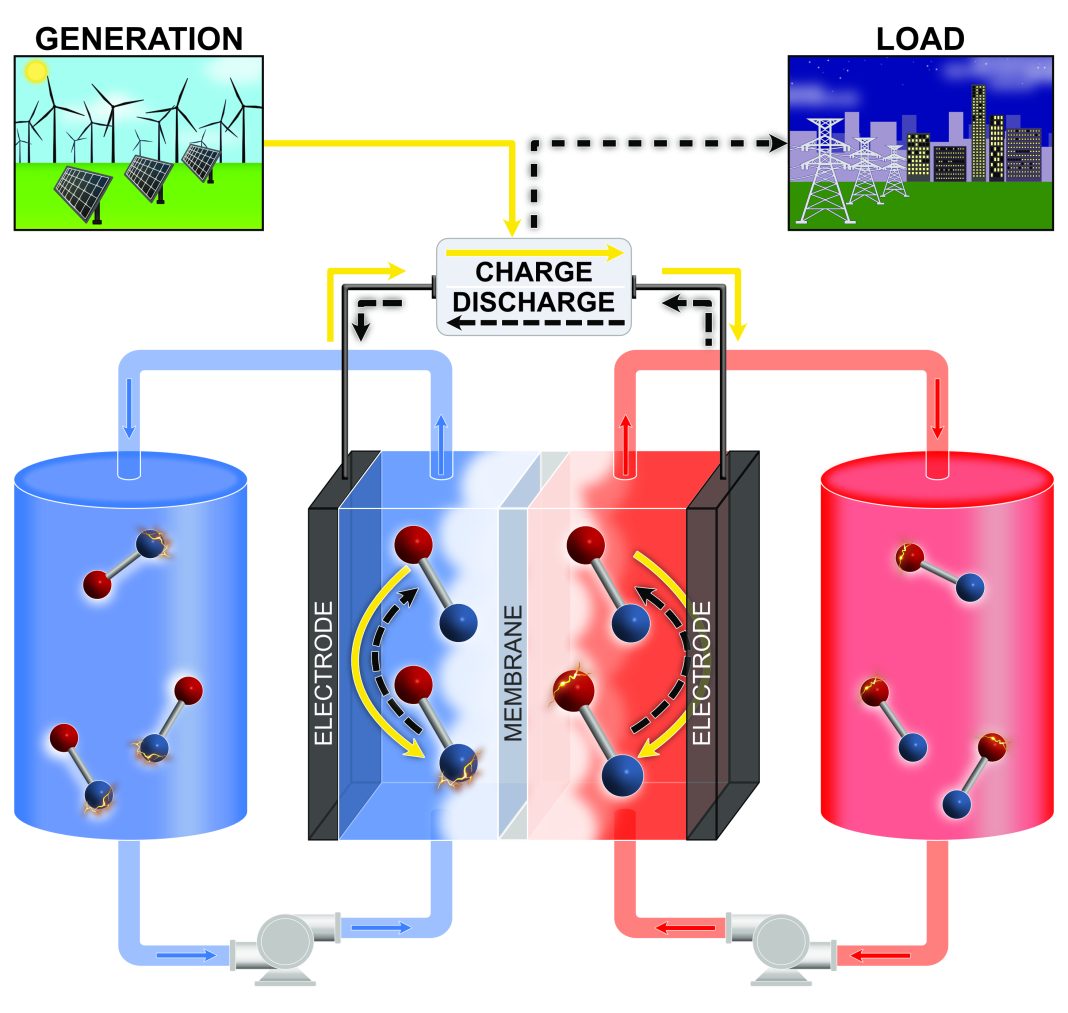
Multiple options for grid-level storage are available, but many of these require particular geographic features, a lengthy permitting/approval process, and/or extremely high capital costs. The use of lithium-ion batteries (LIBs) for grid storage is generally cost-prohibitive, but redox flow batteries (RFBs) may be able to fill the technology gap.
In contrast to traditional batteries, the active materials in a typical RFB are a pair of liquids held in separate storage tanks and pumped through an electrochemical cell, which converts the chemical energy to electrical energy. Much like a gasoline-powered vehicle, the size and design characteristics of the cell (engine) determine the instantaneous power (acceleration), while the size of the fuel tank largely determines the ‘engine’ run duration. This way, power and energy are decoupled in an RFB, providing a scalable, customizable solution. RFB systems based on the relatively scarce metal vanadium are commercially available, but both the high cost of vanadium and the safety of the highly acidic electrolyte remain critical issues. Substantial research efforts are directed toward new flavors of aqueous and nonaqueous RFB chemistries with improved energy content, power capability, efficiency, and service life at a price target of < $70 Wh/kg. Perhaps not surprisingly, there is a focus on low-cost, Earth-abundant, and environmentally benign materials.
Jolt Energy Storage Technologies’ RFB
Jolt Energy Storage Technologies has launched an aggressive development campaign toward a practical nonaqueous RFB that uses no lithium, cobalt, manganese, or vanadium; in fact, no metal-based active substances at all. At the core of Jolt technology is a set of carefully crafted organic compounds called pyridinium salts. Molecular modeling suggested that these compounds would produce relatively high battery voltages, but there was one obvious problem: no one knew how to make them. Jolt researchers recognized that decades-old, obscure scientific literature might hold useful clues and used that information to successfully produce scores of novel chemical candidates. In accordance with calculations, batteries incorporating these new materials in place of more conventional pyridinium salts exhibit much higher voltages (by over 1V!).
A critical factor in designing energy-dense RFBs is the ability to make highly concentrated solutions of the active materials, and this is where the story took an unexpected turn. Intuitively, the new compounds were expected to show good solubility, but the experimental results far exceeded expectations. The Jolt team and their collaborators from Michigan State University were astonished to find that extremely high electrolyte concentrations could be achieved. A machine-learning model employed to find correlations between molecular structure and solubility data surprisingly pointed to attractive forces between neighboring molecules in the crystalline solid. Logically, such attractive forces tend to favor the solid phase, reducing solubility. However, in this case, the weak interactions favored the formation of ‘self-complementary dimers’ in solution; that is, pairs of positively charged pyridiniums swimming side-by-side. This pairing increases solubility dramatically – up to twice the ‘best-case scenario’ concentration.
There was more good news. Because the positive charge centers in the dimers are somewhat buried in the interior of the pair, interactions with solvent molecules are relatively weak. As a result, the new compounds are free to move quite rapidly through solution. This offers three distinct advantages for flow batteries:
- Increased electrolyte conductivity (high battery efficiency);
- Faster rates for charge/discharge (improved power capability); and
- Low viscosity (reduced pumping losses).
Finding a solution to stabilize free radicals
This was great progress, but one daunting problem remained: the charged states of these compounds are free radicals, and the prevailing wisdom held that free radicals tend to be unstable. Were these examples robust enough to survive in real-world conditions? The early answer was a resounding ‘no’, so the Jolt team prepared over 50 new compounds with varying molecular skeletons and again used a machine-learning tool to correlate molecular structural parameters with free radical stability. This approach led to the design of new materials showing an improvement in projected stability by 2000x.
The Jolt team had one more trick up their sleeve: they generated a single species that could function as both of the necessary electrolyte liquids. This allows for the replacement of the ion-exchange membrane ($800/m2) normally used to separate the two solutions in an RFB by a simple porous separator ($2/m2). The switch to an inexpensive separator, coupled with the elimination of the expensive vanadium- based fuel, results in a substantial decrease in overall battery cost.
Is the future organic? The Jolt team certainly believes so and plans to install and demonstrate a fully operational pilot of their all-organic redox flow battery by the end of 2025.