Shani Talice and Benyamin Rosental from Ben Gurion University of the Negev explore how stem cells could help corals recover from stress and environmental damage, addressing the urgent threats of climate change, pollution, and disease to coral reefs
As the lead author, I embarked on a project with an exceptional group of scientists, driven by a shared hope: could stem cells help us restore the world’s coral reefs? This question led me, alongside colleagues from Ben- Gurion University, the University of Miami, Stanford University, and the Hebrew University, into a collaborative quest at the crossroads of marine biology and regenerative medicine.
Why we studied stem cells
Our team was deeply inspired by breakthroughs in medical science, where stem cells have transformed treatments for diseases through their ability to both renew themselves and become different kinds of specialized cells. Yet, it struck us that the tools for isolating and transplanting stem cells had never been brought to Hexacorallia – a group of species that includes sea anemones and stony corals, foundational species for coral reefs. Early meetings in our comparative immunology laboratory always circled back to one question: could these techniques help corals recover from stress and environmental damage?
Urgency and shared vision
As a team, we all felt the urgency. Coral reefs support enormous biodiversity and are vital to people worldwide, but climate change, pollution, and disease are pushing them toward extinction. Although approaches like coral farming have been tried for years, we saw the potential to go further – restoring reefs not just with fragments, but rebuilding them from the cell level up. The prospect of giving damaged corals new life through stem cell transplantation united us in our mission.
Unlocking stem cells in marine life
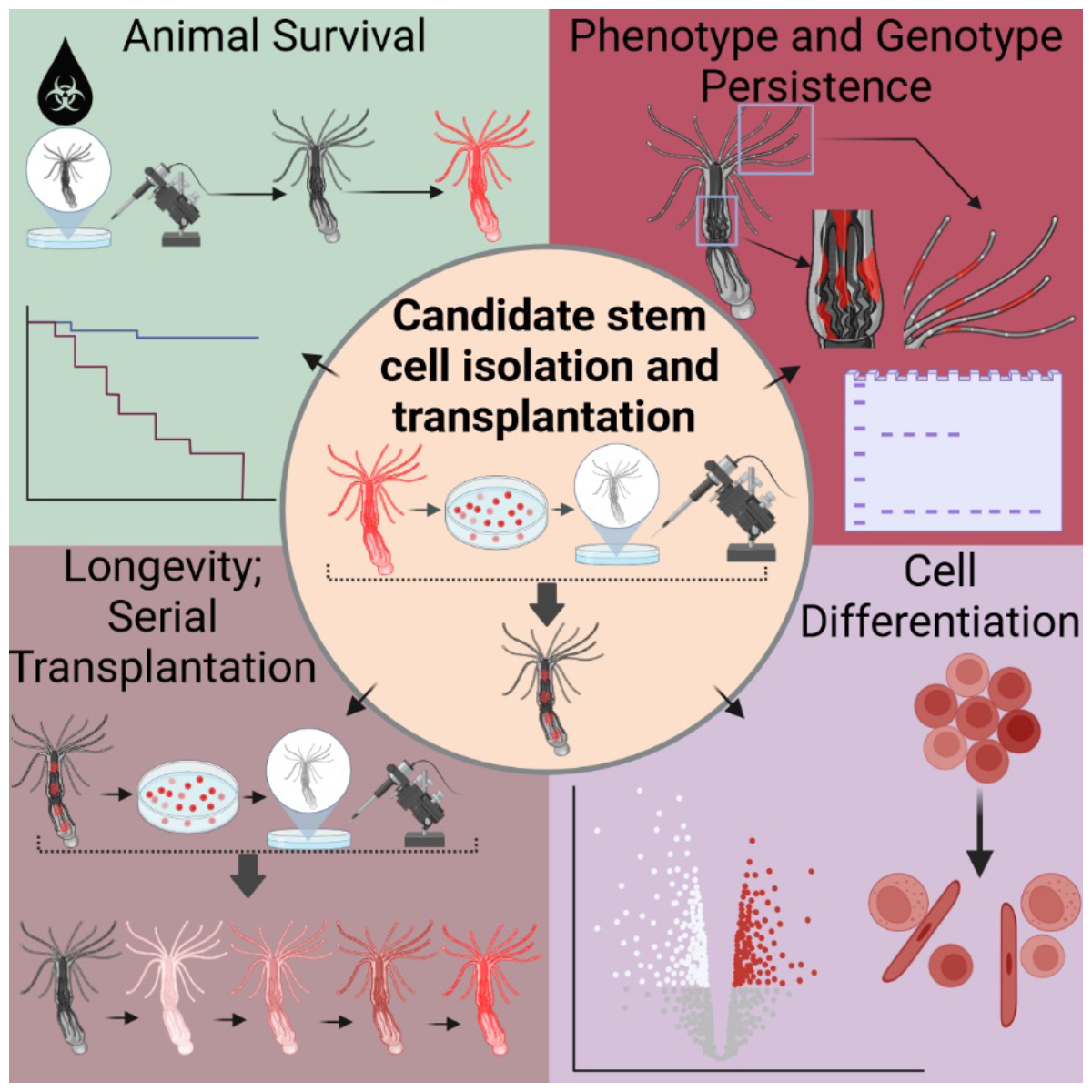
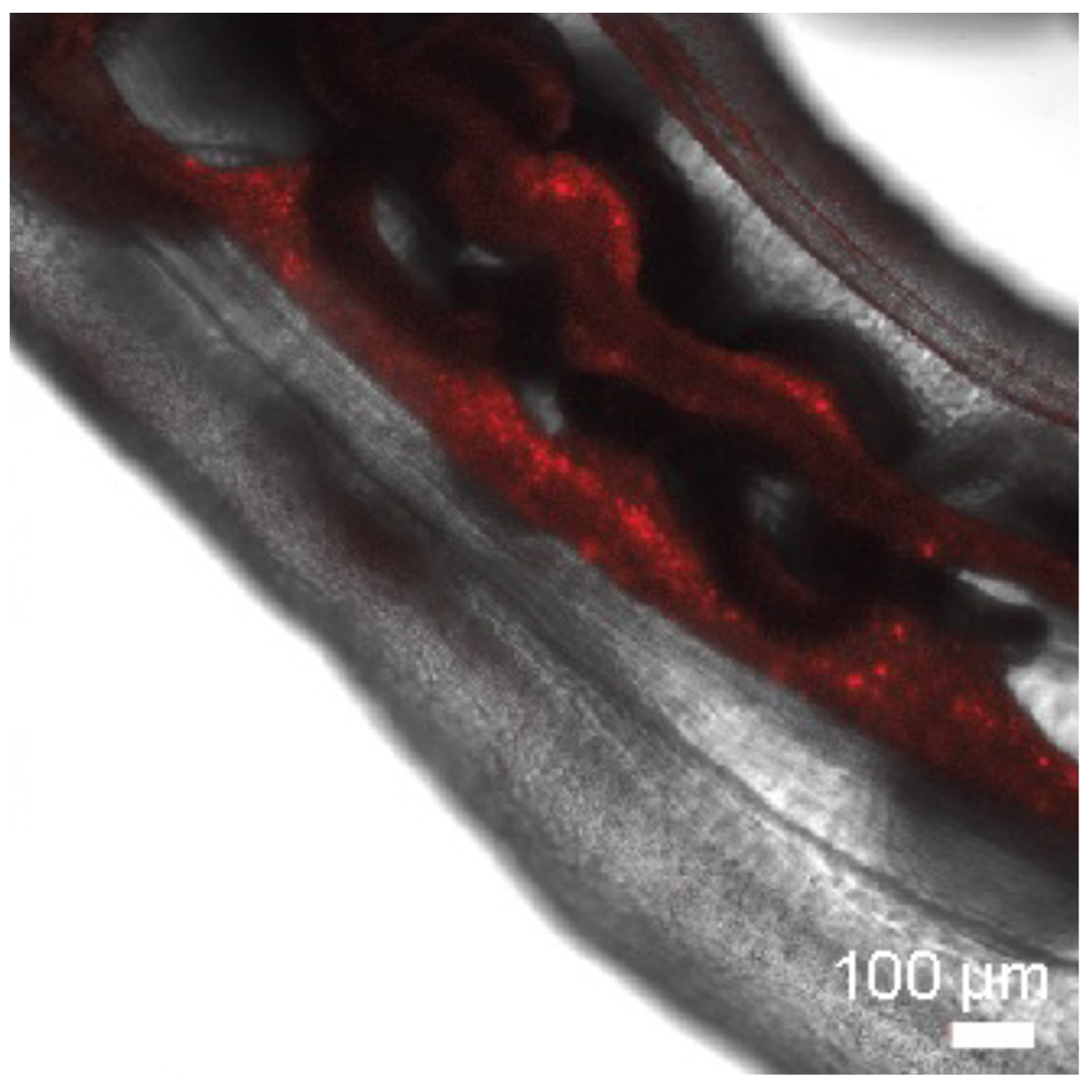
With tremendous input and technical support from our collaborators, we focused our experiments on Nematostella vectensis, the only model sea anemone, closely related to reef-building corals, that glows fluorescently under a microscope. Together, we meticulously isolated cells that exhibited all the defining stem cell properties: self-renewal, survival, and the ability to differentiate into various tissue types (Fig. 1). The most striking results came from our transplantation experiments: when we injected these candidate stem cells into animals weakened by chemotherapy drugs, we watched in awe as recipients grew new tissues and survived otherwise lethal damage (Fig. 2).
Collaboration and technical Innovation
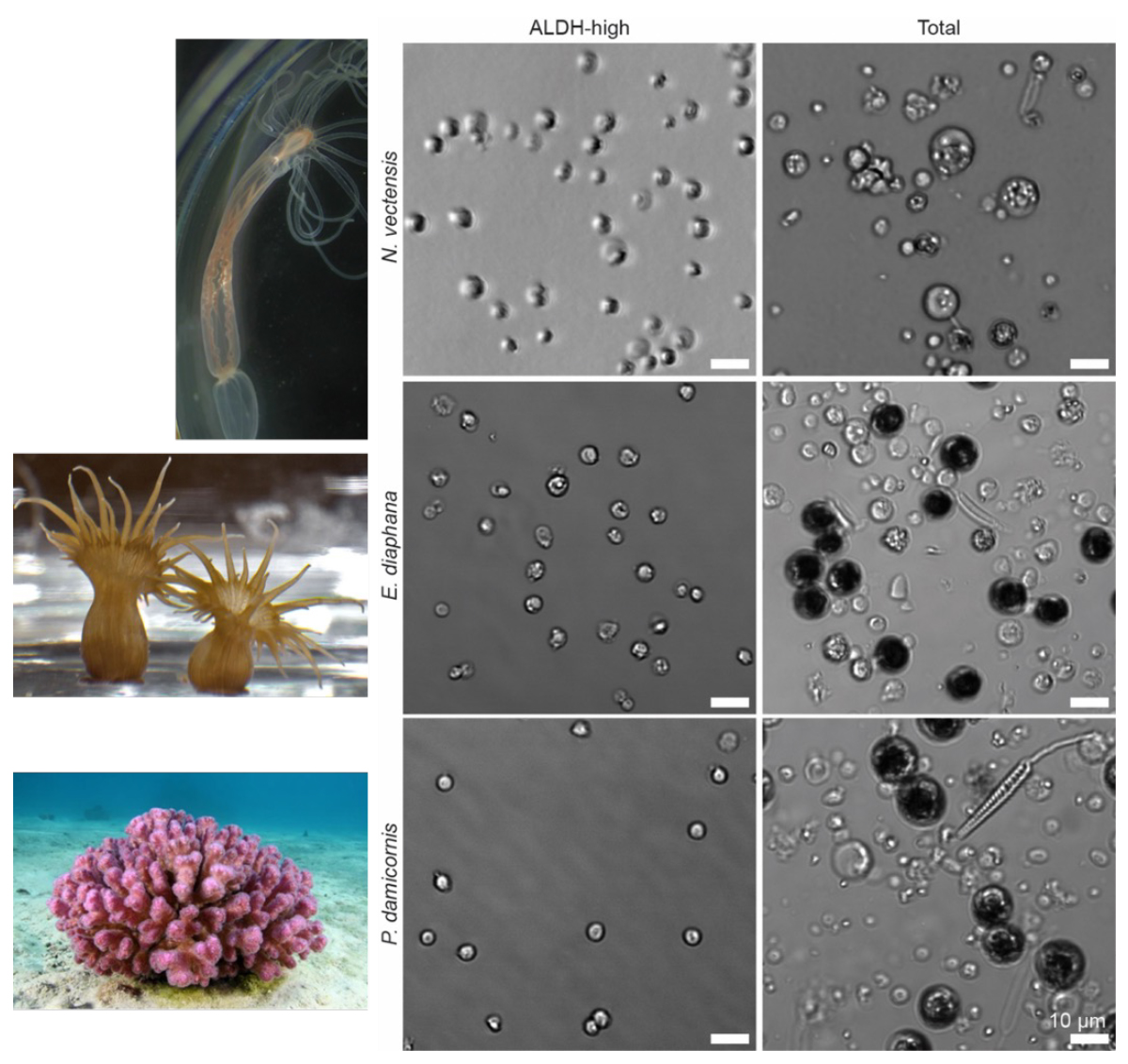
Our work was powered by teamwork at every stage. Colleagues engineered transgenic animals that glowed under the microscope, which allowed us to visually track transplanted cells as they spread through recipient tissues. Bulk RNA sequencing was also performed to verify the existence of the stem cell niche within N. vectensis, confirming the presence of a stem cell-enriched cell population. Within our lab, we used flow cytometry and ALDH markers – recognized tools in stem cell biology – to enrich stem cell populations and carry out serial transplants, verifying the cells’ resilience and their ability to multiply over repeated transplantations (Fig. 3). This level of collaboration across laboratories and expertise enabled outcomes no single researcher could have achieved.
Extending the model: toward coral application
The excitement in our group grew as we demonstrated that similar stem cell enrichment and transplantation could work in stony corals, not just sea anemones. Key contributors from different institutions refined protocols so we could isolate promising stem cells from coral species fundamental to reef ecosystems (Fig. 3). Our combined findings suggest coral conservation could one day harness these cells, offering new hope as climate challenges intensify.
Unique properties of marine stem cells
I want to highlight that our team showed, through molecular and genetic analysis, that the stem cells isolated from the mesenteries (internal tissues) of sea anemones carried hallmark genes linked to regeneration and development. When transplanted, these cells integrated across diverse regions of the host, including the tentacles, demonstrating a remarkable potential to repair and replenish damaged tissue. These discoveries were only possible because of our combined knowledge in genetics, cellular biology, and advanced imaging.
Facing challenges as a team
Despite promising advances, we encountered difficult questions together. Are these true pluripotent stem cells, or are they more specialized? Our current bulk analysis methods have limitations, and some collaborators are now developing single-cell sequencing and lineage tracing approaches that will drive the next phase of research. Importantly, the repeated success in saving damaged animals and integrating donor cells gives us confidence as we move forward collectively.
Building a broad platform for marine science
As lead author, I’m proud that our team has built a foundation for future studies across marine invertebrates. The tools and strategies we developed – validated by cell markers applicable across species – allow researchers everywhere to explore tissue regeneration, stem cell biology, and therapy development in corals and beyond. This widens the impact of our work, supporting efforts to restore diverse coral species that are hard to study using traditional approaches.
Looking ahead: a future built together
If you ask any member of our project what this means for coral reefs, you’ll hear a collective hope: that cellular therapies can be added to the conservation toolbox, alongside restoration, selective breeding, and genetic approaches. The next frontier – regenerating coral ecosystems through cell-based therapy – feels within reach because of our collaboration. As we confront global challenges like climate change and habitat loss, collaboration is more important than ever for securing the future of coral reefs and other ecosystems. By working collectively, scientists can achieve outcomes that would be impossible alone, helping to drive innovation and positive change for our planet.