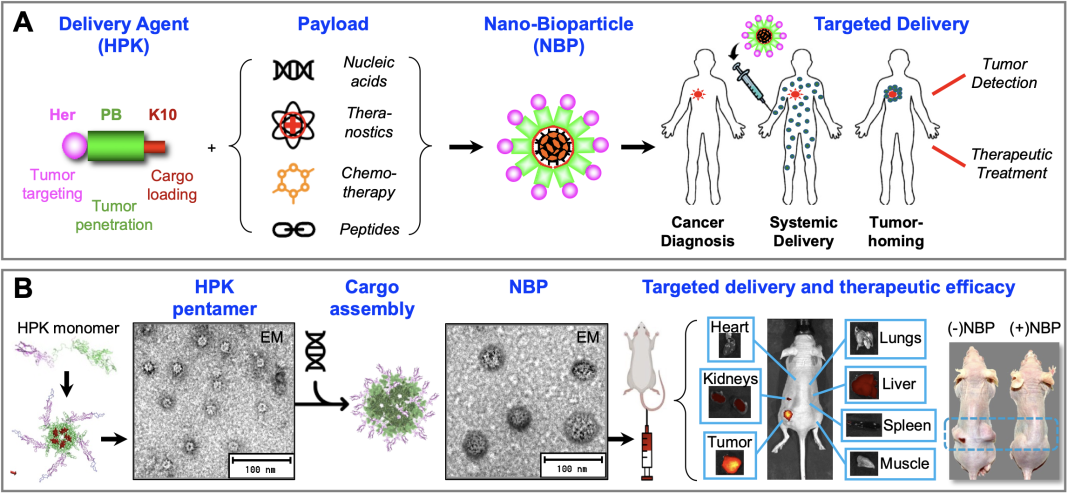
Dr LK Medina-Kauwe developed a bioengineered delivery system to treat resistant and metastatic tumors, highlighting the potential of nano-bioparticles to enhance cancer therapy by targeting specific tumor characteristics and overcoming treatment barriers
Repurposing parts of viruses, growth factors, and nucleic acid-binding proteins has led to the development of a bioengineered therapeutic delivery vehicle being evaluated as a cancer-specific molecular missile. Dr LK Medina-Kauwe combined the cell-binding, membrane-penetration, and intracellular trafficking functions of pathogen proteins and naturally occurring protein ligands to develop a biologically based tumor-homing platform technology designed to self-assemble with and selectively deliver therapeutic molecules to resistant and metastatic tumors. (1-2) These molecular missiles are comprised of nano-sized biologically derived particles, or nano-bioparticles (NBPs; Fig. 1) that can self-assemble with a variety of therapeutic payloads, including:
- Nucleic acids used for the expression of therapeutic proteins, silencing of mutant or cancerous genes, and modulating other intracellular molecular targets; (3-5)
- Drugs and small molecules, such as toxic agents to destroy cancer cells, chemotherapy agents, and other chemical compounds; (6-9)
- Theranostics and other novel compounds to act as multi-functional targeted diagnostics, imaging agents, and/or therapeutics; (10-12)
- Peptide and protein therapeutics to interact with intracellular molecular targets using naturally evolved mechanisms that circumvent the shortcomings of small molecules.
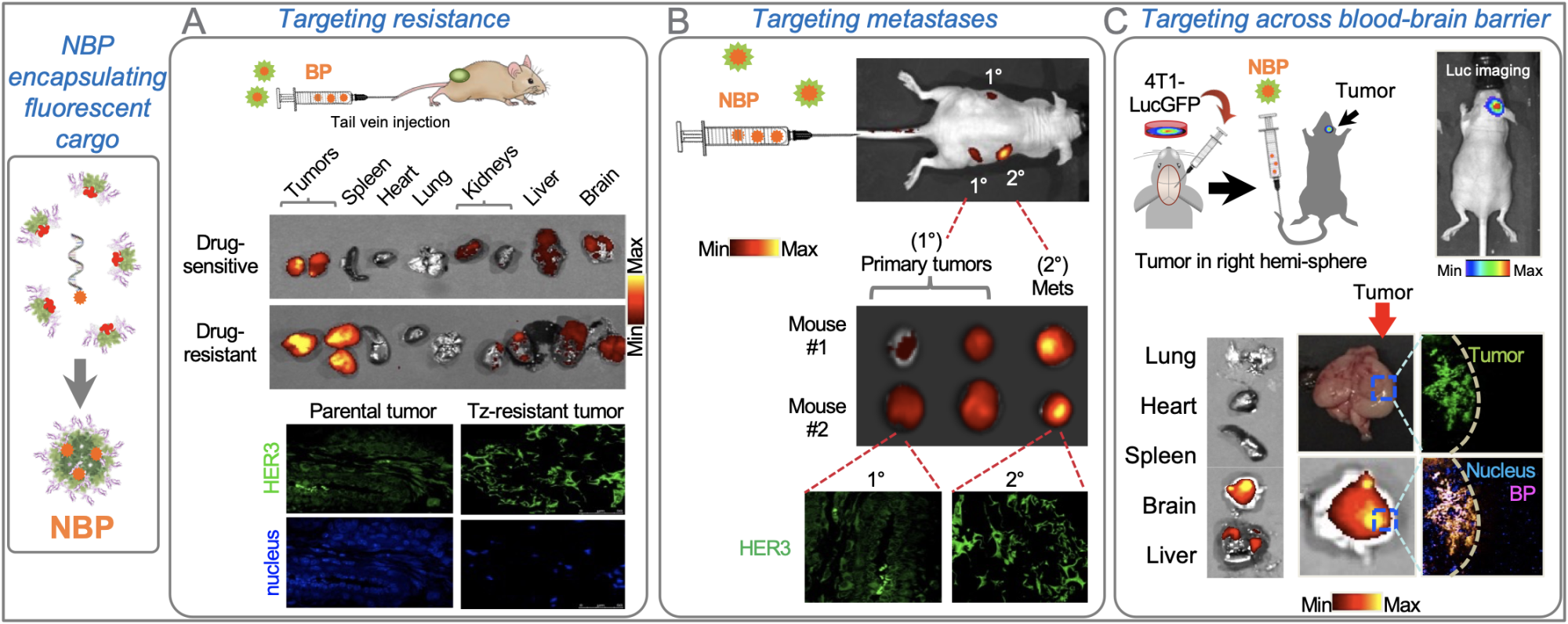
in preferential homing to: A, breast tumors that resist HER2-targeted therapy (which also show higher HER3 compared to parental drug-sensitive tumors); B, secondary breast tumors arising by metastasis (which also show higher HER3 compared to the primary tumors); and C, intracranial triple- negative breast tumors as a model of brain-metastatic breast cancer. In each scenario, tropism was selective for tumors vs non-tumor healthy tissue.
The protein, HPK, developed by Dr Medina Kauwe exploits the cell surface receptor HER3 as a portal for delivering therapeutic cargo, thereby bypassing mechanisms of drug resistance by mimicking an essential ligand that feeds the tumor cell. HER3 is prominently displayed on drug-resistant and metastatic tumor cells. Accordingly, Dr Medina-Kauwe’s research has shown that nano-bioparticles delivered systemically (intravenously) in pre-clinical (mouse) models of resistant and metastatic breast cancer selectively home to tumors (especially resistant and metastatic tumors; Fig. 2, A-B) based on the high levels of HER3 density found on these tumor cells, while bypassing non-tumor tissue. When delivering therapeutic cargo, these nano-bioparticles impart selective tumoricidal activity on the cancer while avoiding toxicity to healthy organs, in contrast to non-selective chemotherapy that affects both tumor and non-tumor tissue.
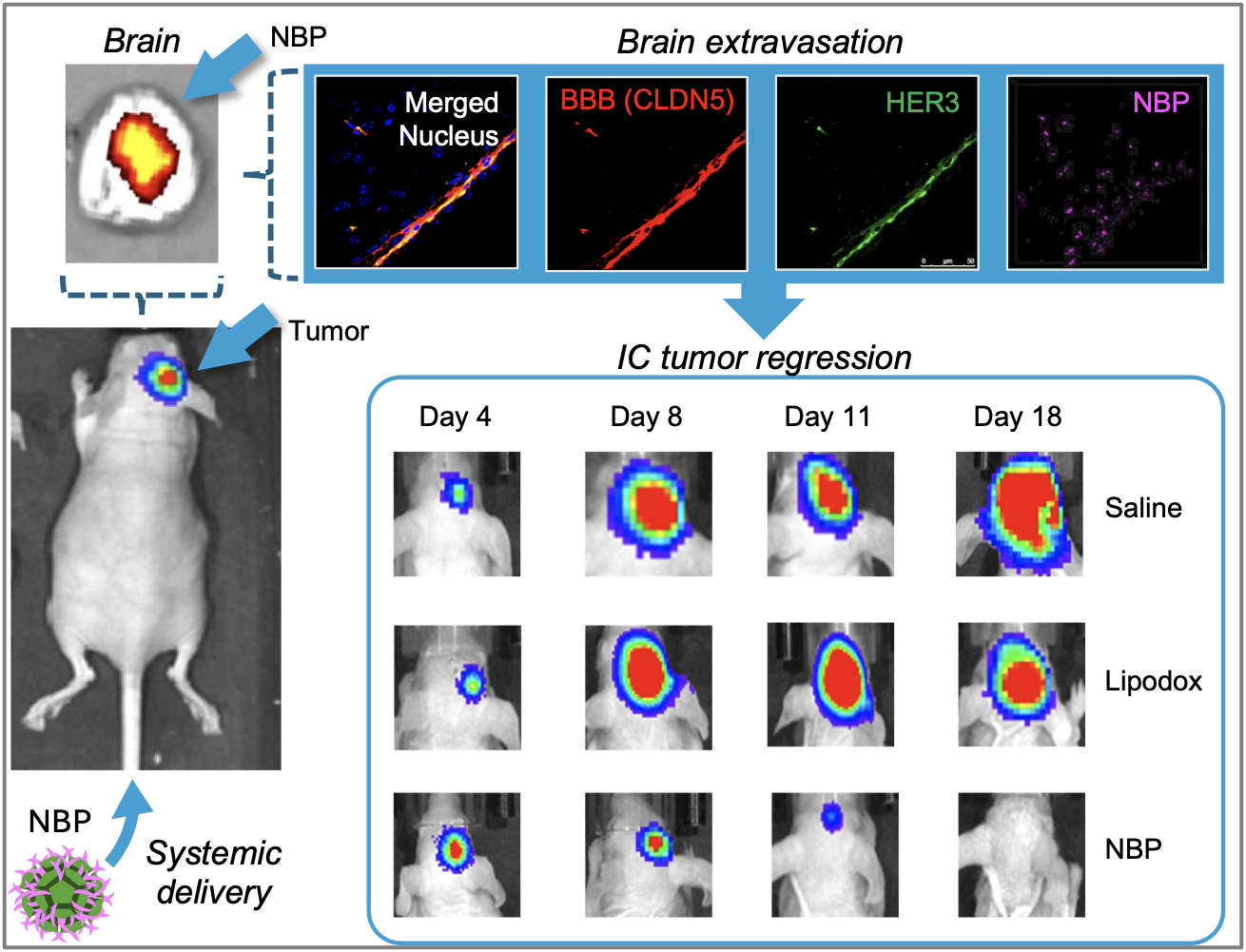
Recently, Dr Medina-Kauwe’s research demonstrated that HER3 is also present on the barrier separating the brain from the bloodstream, known as the blood-brain barrier (BBB), and showed that HER3-homing nano-bioparticles can cross the BBB and enter brain tumors using HER3 to mediate both routes (Fig. 3). (6) Dr Medina-Kauwe’s studies show that nano-bioparticles delivering tumoricidal drugs can home to triple-negative breast cancer tumors in the brain and reduce tumor growth with favorable therapeutic efficacy compared to conventional chemotherapy, which is currently the only recourse for such tumors, albeit with poor clinical outcomes. Importantly, antibody-based therapies and current targeted therapies in the clinic are unable to cross the blood-brain barrier, resulting in a poor therapeutic effect on tumors in the brain.
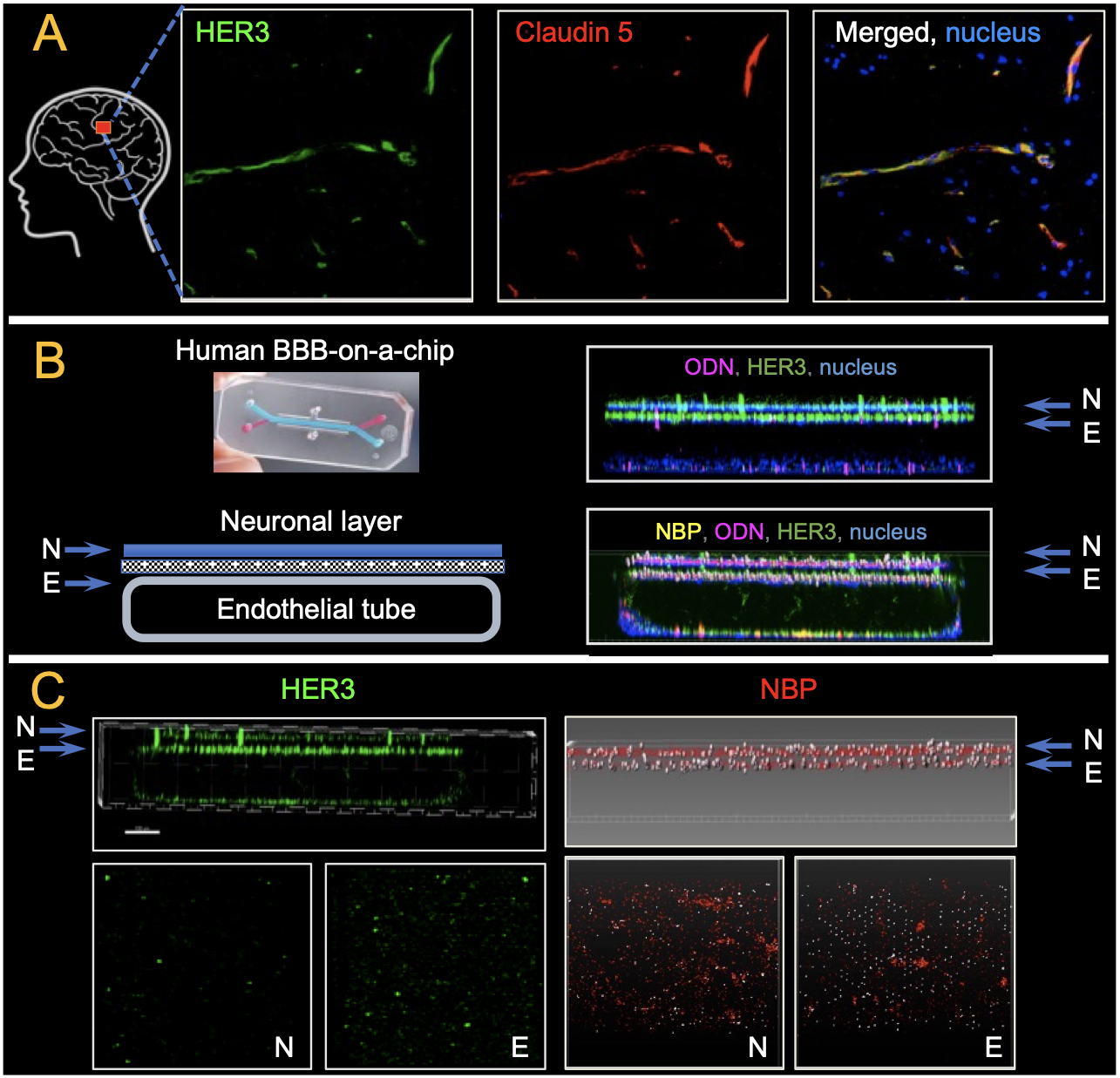
These findings were recapitulated on a human induced pluripotent stem-cell (iPSC) derived blood-brain barrier chip, or BBB chip, which re-creates the human blood-brain barrier by differentiating iPSCs into vasculature overlaid by central nervous system cells (Fig. 4). Like mouse and human brain specimens, the BBB chip displayed HER3 prominently on the vasculature surface that abutted the neuronal tissue. NBPs flowing through the vascular tube crossed the blood vessel barrier and entered the neuronal tissue. These studies demonstrated that both the NBP and its interaction with HER3 were essential for mediating the transfer of cargo across the BBB.
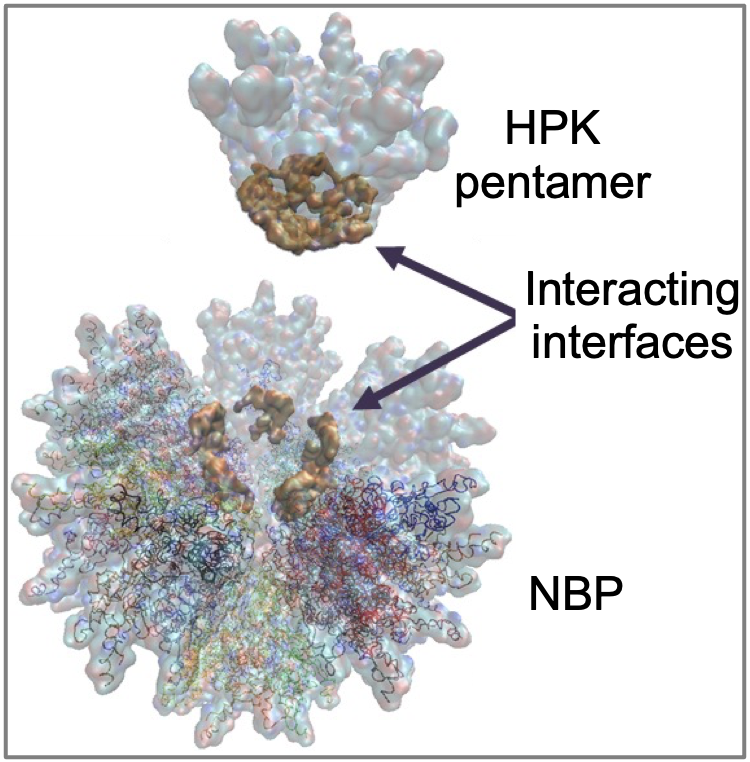
While developing the NBP technology, Dr Medina-Kauwe’s research uncovered previously unknown mechanisms of how viruses penetrate the cells they infect. Specifically, Dr Medina-Kauwe’s interrogation of the adenovirus penton base capsid protein has revealed how the protein’s structure facilitates an ordered self-assembly of HPK into polyhedral particles displaying multiple tumor-seeking probes (Fig. 5) that draw the particles to tumors with high HER3 density and trigger robust entry into the tumor cells to undergo pH-mediated penetration into the tumor cell interior. This research has demonstrated that the protein forms a cork-shaped barrel with a pH-sensing interior pore that responds to an acidifying environment (encountered upon tumor cell entry) by unmasking membrane-destabilizing peptides, thereby enabling tumor cell penetration. This mechanism may overcome key cellular barriers that limit the efficacy of antibody-based therapies and thus may someday bring improved therapeutic efficacy to the clinic.
Dr Medina-Kauwe is an inventor on more than 35 national and international patents affiliated with this research and has previously contributed expertise to two start-up biotech companies. Dr Medina-Kauwe’s research has been supported by the NIH/NCI, the Department of Defense, the Avon Foundation, the Komen Foundation, the American Cancer Society, and other sources of research support. Images were reproduced from original research articles (3, 6, 8) with permissions under the Creative Commons Licenses.