Piezo4Spine is a European project that aims to develop a novel therapy to repair the injured spinal cord, a pathology for which a cure remains elusive. María C. Serrano tells us more
Spinal cord injury (SCI) is a devastating pathology affecting the spinal cord, an essential component of our central nervous system, executing vital instructions, most of which are orchestrated by our brain. According to recent figures from the World Health Organization (WHO), over 15 million people are currently affected by SCI. It is already well-known that the therapeutic window for these patients before the damage and loss of functionality becomes chronic is very limited, often even uncertain.

Current advances on SCI therapies focus on rehabilitation, cell transplantation, drugs, biomaterials, and electrical stimulation, alone or in combination. Although leading to partial sensory/ motor recoveries, chronic functional deficits limit daily living activities and shorten life expectancy in SCI patients, as all these therapies still fail to promote successful and complete neural regeneration at the lesion site and restore all lost functions.
In the context of this societal challenge, the European Project Piezo4Spine is making a remarkable scientific effort to develop an unprecedented multifactorial therapy for SCI. This project brings together seven European partners working closely together on the radical vision of a disruptive platform that enables neural repair by tackling the complexity of this pathology. It includes the Spanish National Research Council as coordinator (CSIC, Spain), the National Hospital for Paraplegics (HNP, Spain), the Italian Institute of Technology (IIT, Italy), the Catholic University of Louvaine (UCL, Belgium), the University of Coimbra (Portugal), and two companies, Black Drop (Germany) and the Austrian Centre of Industrial Biotechnology (ACIB, Austria).
From the scientific perspective, Piezo4Spine has two main focuses. First, the pivotal role of mechanotransduction – the biological process of converting mechanical forces into electrochemical signals – in the functioning and disease of tissues and organs has never been explored in SCI. Second, the true relevance of fibroglial scarring in hampering neural repair. Since January 2023, this four-year project envisions a 3D bio-printed mesh containing therapeutic nanocarriers (one-millionth of a millimeter in size) that act on both fundamental features.
By means of a multidisciplinary consortium combining scientific, technological, clinical, and industrial partners, enriched by their interdisciplinarity and complementarity, we envisage overcoming the limitations of current technologies by targeting multiple cellular pathways involved in neural regeneration after SCI with a balanced combination of therapeutic interventions that optimally promote functional recovery.
Very promising steps
The Piezo4Spine consortium has successfully developed three different kinds of nanocarriers with therapeutic cargos. Intensive work has also been placed on the development of several 3D meshes for loading and controlled delivery of these active nanocarriers. All these materials have been evaluated in vitro using neural, immune, and scar-related cells. The researchers involved in the project have also worked on the design of an active electrode for on-demand cargo release, with an almost completely biodegradable microelectrode array under intensive investigation.
In different preclinical rat models of SCI, the project also examined the effects of motor training on neural repair. Several therapeutic meshes are also under investigation in these models, with both male and female rats included to extend the impact of our findings.
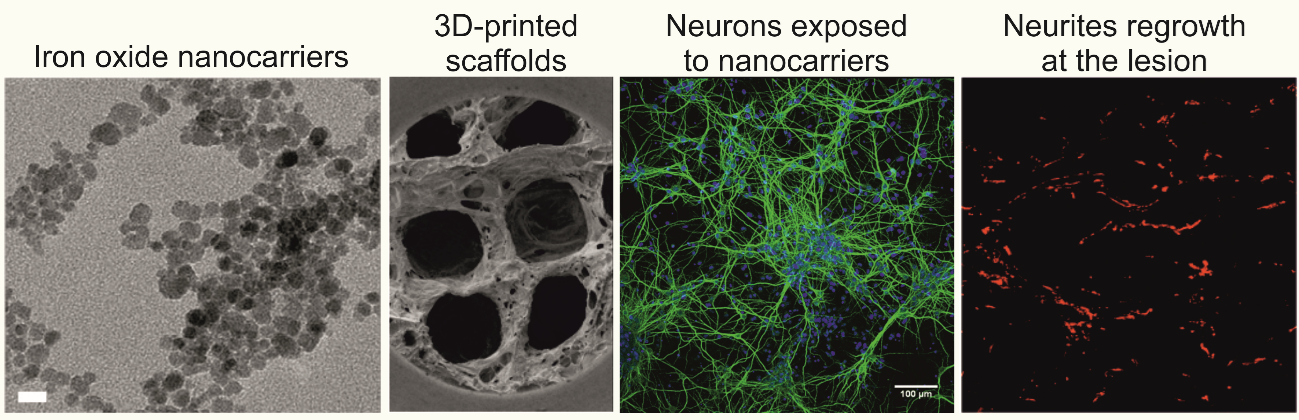
This year, the investigators reported promising data on the neuro-reparative properties of 3D reduced graphene oxide (rGO) scaffolds when chronically implanted in complete transected rats (T9-T10). Electrophysiological recordings from brainstem regions showed activation of a small population of neurons located at the nucleus of the reticular formation and vestibular nuclei in response to electrical stimulation caudal to the injury. Moreover, larger, more abundant blood vessels and longer, more homogeneously distributed axons corroborated the notion that these rGO scaffolds created a permissive environment that facilitated the invasion of functional axonal processes from neurons in brainstem nuclei with motor function. Behavioral tests also evidenced that these scaffolds played an important role in whole-body mechanical stabilization (postural control).
These results hold promise on the path to finding the elusive cure for SCI and encourage further investigation. These radical science-to-technology breakthroughs could, if successful, enable novel technologies and therapies for SCI and many other neural and non-neural pathologies. Intensive research on SCI can make the cure for paraplegia a true reality in a future that may be closer than expected!