Lee J. Martin, PhD from Johns Hopkins University School of Medicine, discusses the pathology of infant hypoxic-ischemic encephalopathy (HIE), and the research he and his team are conducting to understand cell death mechanisms related to HIE and therefore identify new therapies
Brain injury occurring at any time in life is tragic, but when it occurs in babies and children, it is haunting. One cause of brain damage in infants is hypoxic-ischemic encephalopathy (HIE). It arises from reduced brain blood supply, oxygen insufficiency, and nutrient deprivation, for example, because of placental abnormalities and cardiorespiratory arrest. HIE occurs in one to three term gestation infants per 1000 live births. These babies are at high risk of death, and survivors can develop cerebral palsy, or other disorders of movement, epilepsy, and impairments in learning, memory, cognition, and emotional reactivity years later for their entire lives. The individual and family impacts are devastating, and the societal repercussions urgent.
There is only one medical authority-approved treatment for term babies with moderate to severe HIE. It is hypothermia and is done by cooling the infant’s whole body or head to four degrees lower than normal body temperature for about 72 hours. This approach requires specialized, high-resourced hospital facilities. Unfortunately, this treatment works only on about one in seven babies in preventing death; the effects on long-term cognitive and emotional consequences of HIE are limited. The use of neonatal hypothermia has received criticism in low to middle-income countries, and there is concern that rewarming might trigger seizures.
The pathology of HIE
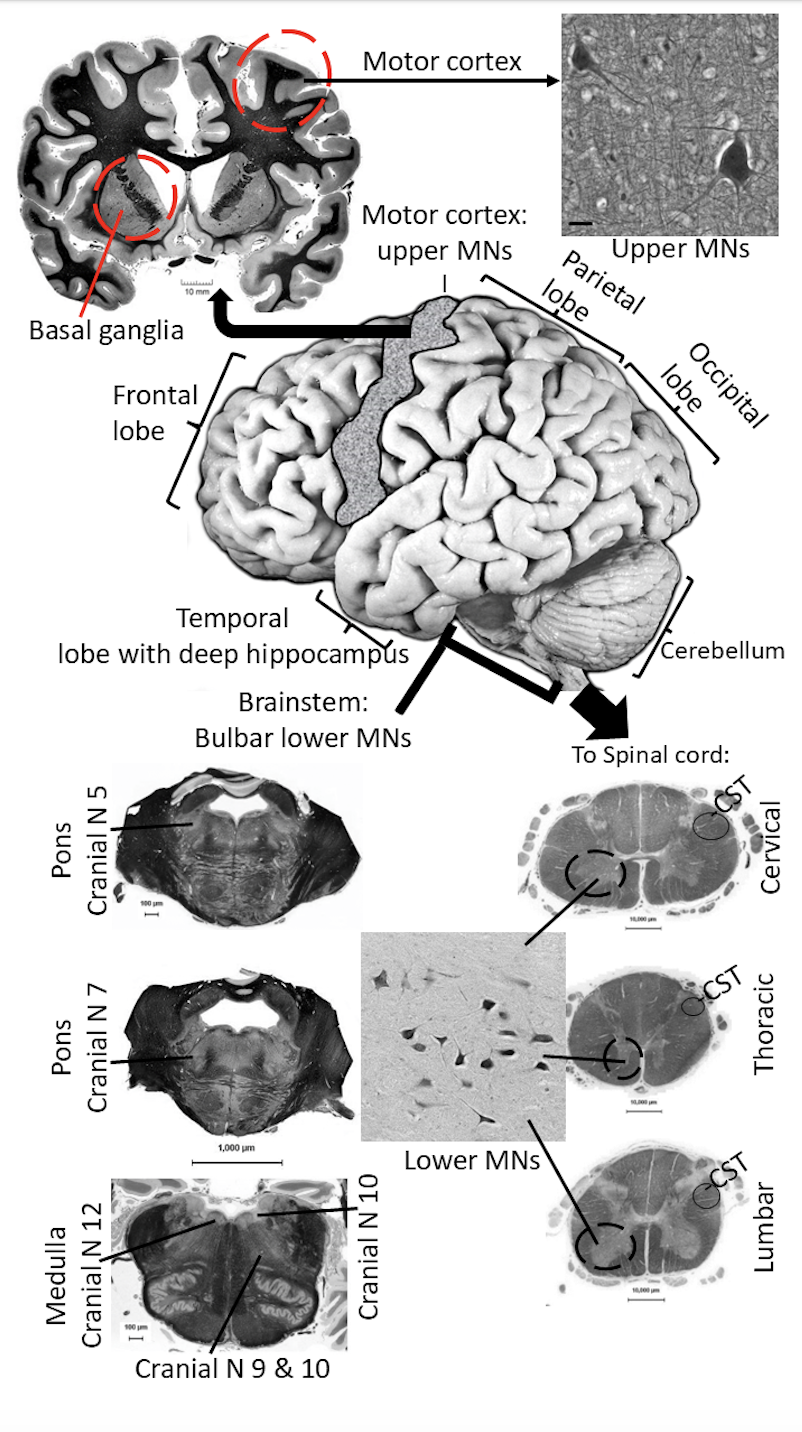
In babies with HIE, the pathology occurs in many brain regions (Figure 1) including the life-sustaining brainstem with the cranial nerve centers (in Figure 1 identified by numbers), the movement planning regions in basal ganglia, and movement enactment regions called the corticospinal tract (CST) and corticobulbar tract that activate the motor neurons in spinal cord and brainstem that control skeletal muscles, the movement coordination regions associated with the cerebellum, and the higher-level learning, memory, and cognition regions of the cerebral cortex located in the different lobes and in the hippocampus (Figure 1).
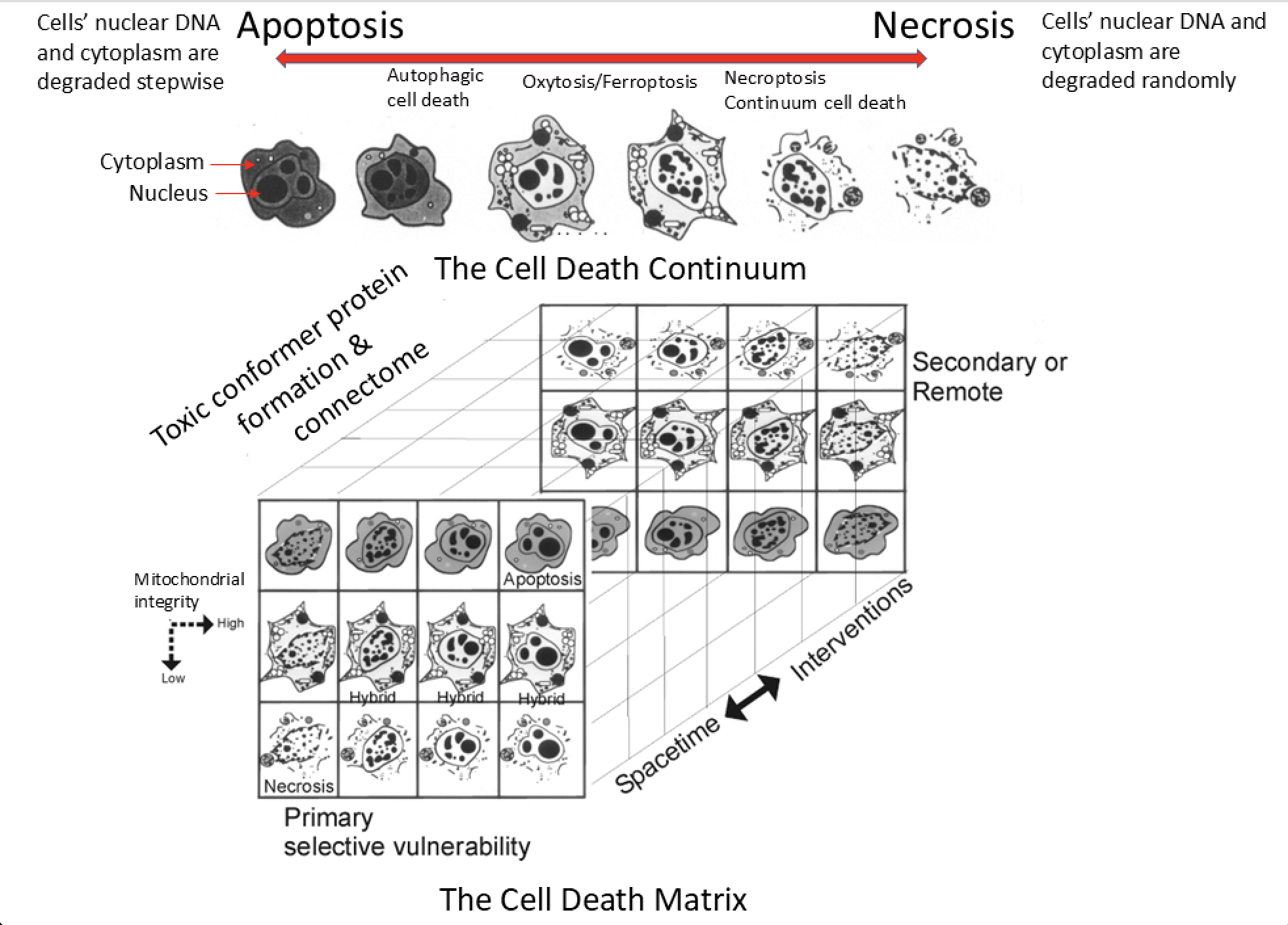
The ways that constituent cells, including neurons and non-neural cells called glia (astrocytes and oligodendrocytes), become injured are amazingly complicated (Figure 2), which makes treating the root causes of HIE very challenging. Lee Martin and his colleagues have been studying the biology of cell death for three decades and have discovered many intriguing aspects of the process that can be summarized in a cell death continuum- matrix diagram (Figure 2). Cells can die rapidly by swelling, disintegration, random internal digestion of macromolecules such as proteins and DNA, and rupturing of critical components. This form of cell death is called necrosis (Figure 2, top right); it is associated with major cerebral swelling and tissue inflammation. In contrast, a tightly regulated form of cell death, called apoptosis (Figure 2, top left), involves more orderly dismantling of the cell and its DNA, utilizing different sets of enzymes in pathways that are described as caspase-dependent and caspase-independent. However, cell death in the brain is not strictly binary and can occur in many forms between necrosis and apoptosis (Figure 2). Moreover, the cell degeneration is asynchronous (the cells die at different times after the insult) and related to the connections among groups of cells called the connectome (Figure 2, bottom left). This biology makes the therapeutic targeting of specific molecular pathways at any specific time with drugs designed to block cell death challenging because of the apparent facile shifting or switching of cell death pathways and the asynchrony of the cell degeneration. This cellular enabling is not only problematic for neonatal HIE but also for neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis.
Advancing HIE research
Recently, Martin’s laboratory has made unique observations in the brains of infants that died from HIE and had consent for an autopsy. The neuropathology in infants who received or did not receive hypothermia treatment was compared. Their results gave proof for the cell death continuum (Figure 2): hypothermia shifted the pattern of brain cell death from widespread necrosis to apoptotic variants with apparent preservation of brain microstructure and less inflammation, but brain cell degeneration was still prevalent and widespread even with the cooling.
Drawing on our prior work on experimental animal models of neonatal brain injury, Martin’s group then searched for evidence of proteinopathy in the neonatal HIE cases. Proteinopathy is a molecular pathology of proteins. Normal proteins become damaged, modified, or cleaved by free radicals such as hydrogen peroxide, superoxide, or peroxynitrite, or by enzymes called protein kinases or caspases. These altered proteins can adopt an abnormal three-dimensional structure or folding or cellular localization that converts them into pathologically toxic entities called toxic conformer proteins (Figure 2 bottom left). Some of these newly formed toxic proteins can serve as templates to promote the misfolding of their normal protein counterparts, leading to abnormality and spreading from one cell to another through the connectome (Figure 2, bottom). These are properties of prion proteins that are known to cause profound neurodegenerative disease in humans, such as Creutzfeldt-Jakob disease, scrapie in sheep, bovine encephalopathy in cattle, and chronic wasting disease in deer. In the infant HIE brains, Martin’s group found for the first time abnormal prion-like forms of α-synuclein, superoxide dismutase, and prion protein. However, unlike the typical prion diseases, these proteins are not infectious or transmissible from person to person by contamination, but they might be transmissible from brain cell to brain cell in an infant with HIE.
This idea of prion-like protein formation in the brain of infants with HIE could be transformative in our understanding of infant and childhood neurological disease. It is consistent with brain imaging studies using structural and functional MRI that show specific brain networks or circuits are damaged (Figure 1) and that children surviving HIE can have lifelong neurological abnormalities.
Martin’s group did parallel experiments using human induced pluripotent stem cell (IPSC) derived neurons and oligodendrocytes grown in a culture dish to simulate infant HIE, and on a newborn pig model of HIE. They found accumulation of similar abnormal proteins after injury. These models can now be used to study how the toxic proteins are formed and how to prevent their formation, which will help develop new therapies for infant HIE. Much more work needs to be done, and research funding needs to be gleaned to potentially transform this idea into a therapy.