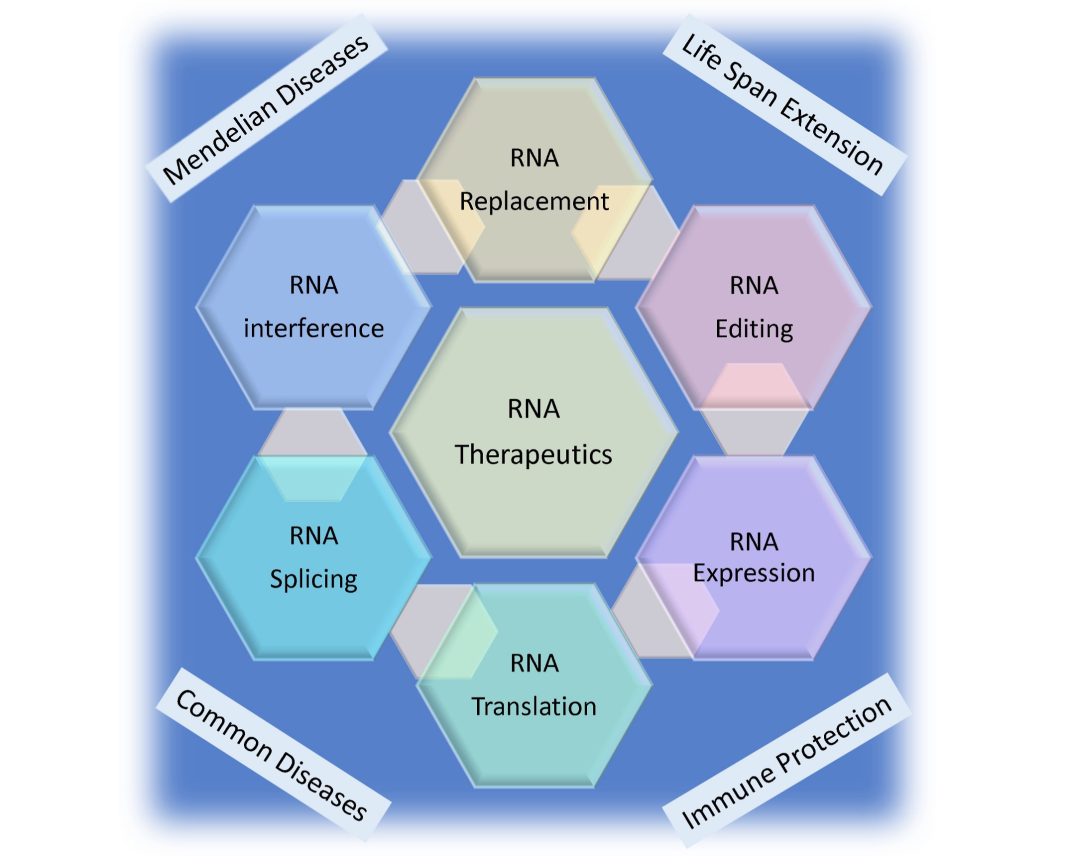
Alan Herbert, Founder and President of InsideOutBio, discusses the significant advancements in RNA therapeutics, highlighting their role in supporting public health and their transformative potential in modern medicine, particularly for addressing genetic conditions and cancer
Currently, much attention is focused on healthy life-span extension. We forget that in 1900, the life expectancy for many countries was around 40, not the 70 years or more common today. As part of our cultural amnesia, we overlook the threat that pathogens have historically posed to killing individuals at a young age. The advances in life expectancy have come from improvements in water and sewage management, the elimination of insect vectors, such as fleas, lice, and mosquitoes, that transmit infectious agents, and from universal vaccination. The SARS-CoV pandemic was a grim reminder of that earlier time when 10-50% of the population died. In the current era, increasingly dense urbanization and rapid worldwide travel allow the rapid spread of similar human pathogens.
The SARS-CoV epidemic was notable in another way. It accelerated the first large-scale deployment of RNA medicines. The vaccine developed was specific, safe, stable, and suitable for serial shots. It was a product of the genomic age, where any vaccine can be designed by selecting an immunogenic sequence from a pathogen’s genetic code. Injecting the RNA encoding that protein into a host then elicits antibodies that can defeat the infectious threat. All that is necessary is to make the RNA and broadly distribute the vaccine. There is no need for the specialized production lines previously used to make protein vaccines or to create cold chains for their delivery. Like all technological innovations, the rapidity of this advance has engendered resistance to change.
The current reemergence of measles in unvaccinated children is an example of what happens when the past ravages of infectious diseases dim in the collective conscience.
Developing targeted therapies for genetic conditions and cancers
The next generation of RNA medicines is under development to replace missing or defective proteins arising from single-gene diseases. These approaches required further innovation to deliver the tissue to where the missing protein was needed. The problem was solved by encapsulating the RNA medicines in small lipid particles covered with antibodies that bind specifically to and are internalized by the target cell. For example, antibodies to CD117
help deliver hemoglobin RNA to blood stem cells in patients with thalassemia. In contrast, antibodies to the transferrin receptor allow RNA medicines to cross the blood-brain barrier to deliver new RNA therapeutics for neurological diseases.
Further innovations are allowing a modular approach to the manufacture of delivery systems. A generic capsule can be built with docking sites that bind to a constant region common to many different antibodies. The selection of the antibody used for the final coat can then be based on the intended application. Dosing by this approach depends on the number of times the RNA is translated in the target cell. Most importantly, the dosing schedule reflects the stability of the protein product once it is produced by a cell. For proteins like hemoglobin replacements for thalassemia, this can be as long as the 180-day life span of a red blood cell.
The third generation of RNA medicines aims to provide cures by delivering novel proteins to cells that defend against cancers or to eliminate cells that have become dysfunctional.
For example, RNAs that enable the specific targeting of cytotoxic immune cells to the cancer cells are being designed. These RNAs encode proteins that recognize the abnormal features present on the surface of a cancer cell. These therapeutic outcomes can be achieved without any alteration to the patient’s DNA sequence, as happens with DNA-editing approaches.
Quite independently, a different class of RNA medicine based on short RNA sequences of 10-40 bases long has been developed. The therapeutics are referred to as oligonucleotide therapeutics (ONTs) and bind specifically to the sequence of bases in the cellular RNA target. Currently, there have been 22 of these drugs approved by the FDA and EMT, although three have been subsequently withdrawn from the market, usually for competitive reasons . There were several innovations behind these successes. Many of the chemistries developed increase ONT stability in storage and in the body to prolong their duration of action. Other modifications prevent the ONT from triggering immune defenses designed to protect against RNAs produced by pathogens. The current generation of ONTs is well tolerated and is non-toxic.
The delivery of ONTs to their site of action also required many innovations. Due to their stability, high therapeutic levels in cells can be obtained by the slow release of the ONTS into the blood from subcutaneous injection sites. In some cases, only semi-annual dosing is necessary to maintain a therapeutic effect. Also, attachment of small molecules capable of binding to a receptor on the target cell can greatly enhance delivery and reduce dosage. For example, the addition of the small sugar GALNac allows efficient delivery of therapeutics to the liver. Nanoparticle delivery of ONTs is also more efficient than with other RNA medicines, as each capsule contains more copies of the therapeutic due to their small size. Whether ONTs can be delivered orally is currently an aspirational goal for the field.
ONTs act in several different ways to eliminate or modify disease-causing RNAs. The specificity of ONTs comes from their sequence. ONTs produce a therapeutic effect by utilizing well-understood cellular RNA processing pathways. Some ONTs prevent the translation of RNA into proteins. Others alter how RNAs are processed. As a result, cells function better. Other ONTs exploit the enzymatic machinery in the cell. They form complexes with enzymes that recycle and can modify many different copies of the target RNA. These catalytic processes ensure that many, if not all, of the targeted RNAs are hit. Examples include the use of the RNA interference machinery (RNAi) and the RNaseH enzyme to eliminate specific RNAs from a cell.
A new class of RNA-targeted therapy has just entered the market. These allow the recoding of the mRNA to change just one residue in the protein produced. The approach can achieve the same outcome as gene editing, but without irreversibly altering DNA. The amino acid change may correct a genetic variant that causes disease, or modify the amino acids that are used by various signaling pathways to control a protein’s activity or turnover. This approach uses the ADAR family of editing enzymes.
From traditional ‘magic bullet’ approaches to precise, individualized treatments
This new generation of therapies builds on the innovations used to develop RNAi. The technology now exists to make bespoke therapeutics for each patient, the so-called ‘n=1’ therapeutic. Alternatively, large-scale editing of RNAs is also possible, where an entire section of a defective mRNA is replaced. This approach treats many patients, regardless of where the patient’s disease-causing variant is found in the targeted RNA. Other methods involve using microRNAs to reprogram a target cell. These small RNAs act on many different genes in a pathway all at once, a discovery that was awarded the Nobel Prize in 2024. It is also possible to program DNA with small DNAs to change the expression of specific genes without altering the DNA sequence, as described in a recent paper by InsideOutBio.
These new therapies are becoming available at scale. Many innovations have led the way. It is estimated that RNA medicines designed to treat common diseases that affect over 10% of the population would require a production run exceeding one million kg of therapeutic annually. Such capacity is rapidly coming online. The developments signal that we are moving from the age of ‘magic bullets’ that take out disease with a designer drug, to precise targeting informed by the individuality of the RNAs we make. The new approach promises less collateral damage and cheaper delivery of medicines to individuals in need.