Dr Erin C. Berthold from Planted in Science Consulting LLC discusses the uses and perceptions of botanical medicines, emphasizing the necessity for coordinated global efforts to understand and regulate these substances to ensure their safe integration into healthcare
Botanicals have long been used in traditional medicine practices. In developing nations, particularly in Africa, up to 80% of the population relies on traditional medicine as their primary source of care. (1) This historical use of botanicals as medicines also profoundly shaped modern pharmacology, with botanical compounds serving as frameworks or direct sources for roughly one-third of all drugs approved by the U.S. Food and Drug Administration (FDA). (2)
The growing demand for ‘natural’ remedies
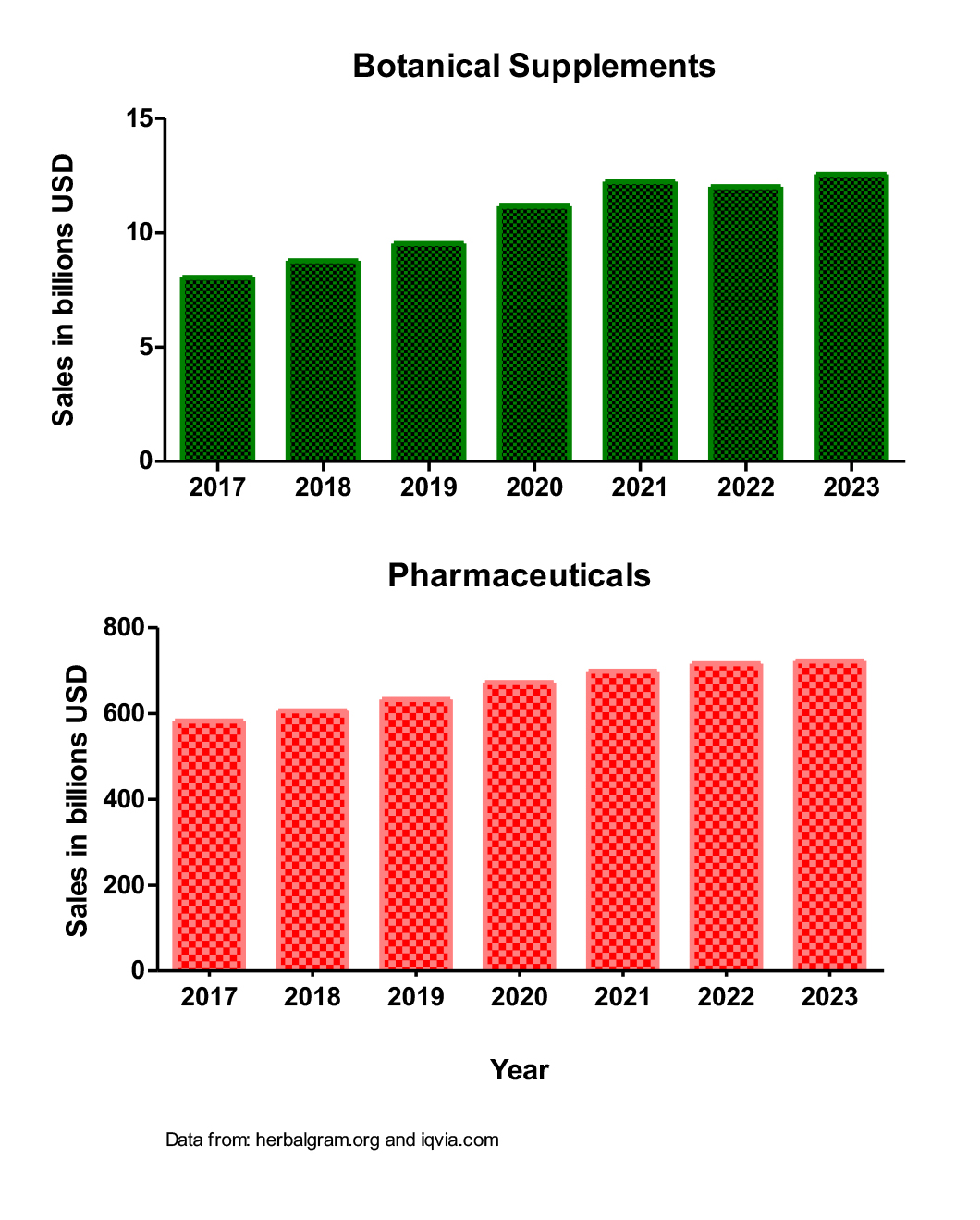
Today, however, the landscape of botanical medicine is undergoing a significant transformation. A surge in wellness trends and a growing consumer preference for ‘natural’ products has led to a boom in their use in developed countries. This shift, combined with a steady increase in the use of prescription medications, is creating potential for botanical-drug interactions. Sales data show that from 2017 to 2023, there was an increase of over $4 billion in sales of botanical supplements in the United States, while at the same time, pharmaceutical sales grew by over $100 billion (Figure 1).
Safety concerns surrounding botanical supplementation
A pervasive misconception about botanicals is that they are inherently safer than pharmaceuticals. This belief is based on the long history of traditional use of plant medicines, the accessibility of botanical supplements, and a bias against ‘synthetic’ chemicals. This leads many individuals to use botanicals concurrently with their prescription medications without informing their healthcare providers. This co-administration of botanicals and pharmaceuticals is not a niche behavior. A survey of US participants revealed that 38% used herbals alongside prescription drugs, with a concerning prevalence among adults over 70 – a demographic particularly vulnerable to adverse effects from polypharmacy due to altered metabolism. (3) Compounding this issue, as many as two-thirds of individuals do not disclose their botanical supplement use to their physicians, creating a significant information gap in patient care. (4)
While the pharmaceutical industry is held to a rigorous standard, requiring extensive pre-market research into drug-drug and food-drug interactions, botanical supplements are often governed by different, far less stringent rules, and issues are often only recognized in post-market surveillance. This regulatory disparity leads to a marketplace where botanical products lack standardized potency, clear instructions, and any meaningful data on their potential interactions with prescription medications.
Botanicals are chemically complex, containing a multitude of compounds that may alter the effects of a co-administered drug. In some cases, they may increase the effects of a prescribed medication, leading to adverse events, a particularly dangerous scenario for drugs with a narrow therapeutic window. Conversely, they may decrease the efficacy of a drug, which can be life-threatening or life-changing when the medication is for a critical condition like cardiac disease or contraception. (5)
The clinical consequences of this regulatory gap and public misconception are becoming increasingly visible. One trend is the rising incidence of drug-induced liver injury (DILI) due to herbal and dietary supplements. While DILI from botanicals is often idiosyncratic, its prevalence is growing. Data from the Drug-Induced Liver Injury Network (DILIN) shows that the percentage of DILI cases attributed to herbal and dietary supplements soared from 7% in 2004-2005 to 20% in 2013-2014. (6)
Botanicals and research gaps
Despite the growing prevalence and clear risks, research into botanical-drug interactions has not kept pace. In fact, a search of the biomedical database PubMed reveals a decline in published clinical trials on the topic. In the ten-year period from 2002 to 2012, there were 51 published randomized controlled clinical trials on herb-drug interactions, but in the subsequent decade, that number dropped to just 25.
The time for a globally coordinated effort to understand and regulate botanical medicines is long overdue. As these products become more integrated into the daily lives of millions, it is imperative that we bridge the gap between historical tradition and modern pharmacology. We must move beyond the idea that ‘natural is safe’ fallacy and dedicate the necessary resources to research, regulation, and education. This will require a collaborative approach that brings together researchers, regulatory agencies, and healthcare providers to develop standardized methodologies and a centralized database for reporting and tracking these interactions. Only by doing so can we ensure that the use of botanical medicine is not just a nod to tradition, but a truly safe and informed choice.
References
- World Health Organization. (2002). WHO Traditional Medicine Strategy 2002-2005. Geneva, Switzerland.
- Newman, D. J., & Cragg, G. M. (2020). Natural Products as Sources of New Drugs over the Last 40 Years. Journal of Natural Products, 83(3), 770-803.
- Posadzki, P., Watson, L. K., & Ernst, E. (2012). Prevalence of herbal medicine use in the US: A systematic review. European Journal of Clinical Pharmacology, 68(11), 1395-1402.
- Kaptchuk, T. J., & Eisenberg, D. M. (2001). The persuasive placebo: A re-examination of the relationship between mind, body, and drug. New England Journal of Medicine, 344(23), 1735-1744.
- Zeller, A., & O’Brien, R. (2009). The potential for herb-drug interactions with cardiovascular medications. Journal of Cardiovascular Nursing, 24(4), 263-270.
- Navarro, V. J., Barnhart, H., Bonkovsky, H. L., Davern, R. J., Fontana, H. R., Jones, D. B.,… & the DILIN Network. (2014). Liver Injury from Herbal and Dietary Supplements in the US. Hepatology, 60(2), 585-594.


