PCB production has been banned for 4 decades, so why are these forever chemicals resurfacing as contemporary contaminants of significant environmental health concern?
Polychlorinated biphenyls, which are more commonly referred to as PCBs, are a structurally related family of manmade chemicals of long-standing notoriety in environmental health. PCBs were produced worldwide in large quantities from the late 1920s to the late 1970s for use in diverse industrial applications and commercial products. However, as early as the 1930s, concerns about adverse health effects associated with occupational exposures to PCBs began to surface. By the 1960s and 1970s, it had become apparent that PCBs, which are highly resistant to degradation, were accumulating in the environment, including the human food supply, and were readily detected in samples collected from the general population, including breastmilk. In parallel with these reports of environmental persistence and widespread human exposure, data had emerged strongly linking PCBs to increased risk of cancer. These observations prompted legislation banning the production of PCBs in the United States (U.S.) in 1979, and in the United Kingdom (U.K.) in 1981, which was subsequently adopted internationally in 2001 by the Stockholm Convention on Persistent Organic Pollutants (POPs).
Over the two decades immediately following the initial bans on PCB production, environmental health scientists documented decreasing levels of PCBs in the environment, toxicologists identified the biological mechanisms by which PCBs cause cancer, and regulatory scientists established generally “safe” levels for PCBs based on cancer as the endpoint of concern. Thus, many believed that with the continued abatement of environmental PCBs as prescribed by the Stockholm Convention, the human health risks associated with PCBs would quickly become a problem of the past. Unfortunately, this prediction has not become reality. Rather, recent studies indicate that (1) environmental levels of PCBs are no longer decreasing, and may in fact be increasing; (2) novel PCBs that were not part of the industrial mixtures synthesized prior to the production ban are being detected in the environment and in human tissues, and experimental studies have shown these non-legacy PCBs are toxic; and (3) PCBs have been shown to cause non-cancer health effects via mechanisms other than those on which regulatory decisions are currently based.
So why didn’t the PCB mitigation plan work? Below, we discuss the data that is triggering renewed urgency for research to understand and mitigate the risks these forgotten “forever chemicals” pose to the environment and human well-being.
What are Polychlorinated Biphenyls (PCBs)?
Commercial manufacture of PCBs began in the 1920’s. In the United States and United Kingdom, PCBs were synthesized and marketed primarily as Aroclor® mixtures whose degree of chlorination was identified by a four-digit designation (e.g., 1248, 1254, 1260), with the first two digits referring to the number of carbon atoms in the biphenyl backbone, and the last two digits identifying the percentage of chlorine by mass in the mixture. Similar PCB mixtures were synthesized worldwide and identified under several trade names such as Clophen® (Germany), Phenclor® (France) and Kanechlor® (Japan).
PCBs have many desirable physicochemical characteristics, including low flammability, chemical stability, and electrical insulating properties. Thus, PCB mixtures were widely used as dielectrics, coolants, lubricants, and plasticizers in electrical transformers, capacitors, fluorescent light ballasts, hydraulic equipment, and lubricating and cutting oils. They were also broadly incorporated into a variety of commercial products such as thermal insulation material, paints, cements, caulking compounds, pesticides, flame retardants, varnishes, adhesives, carbonless copy paper and newsprint.
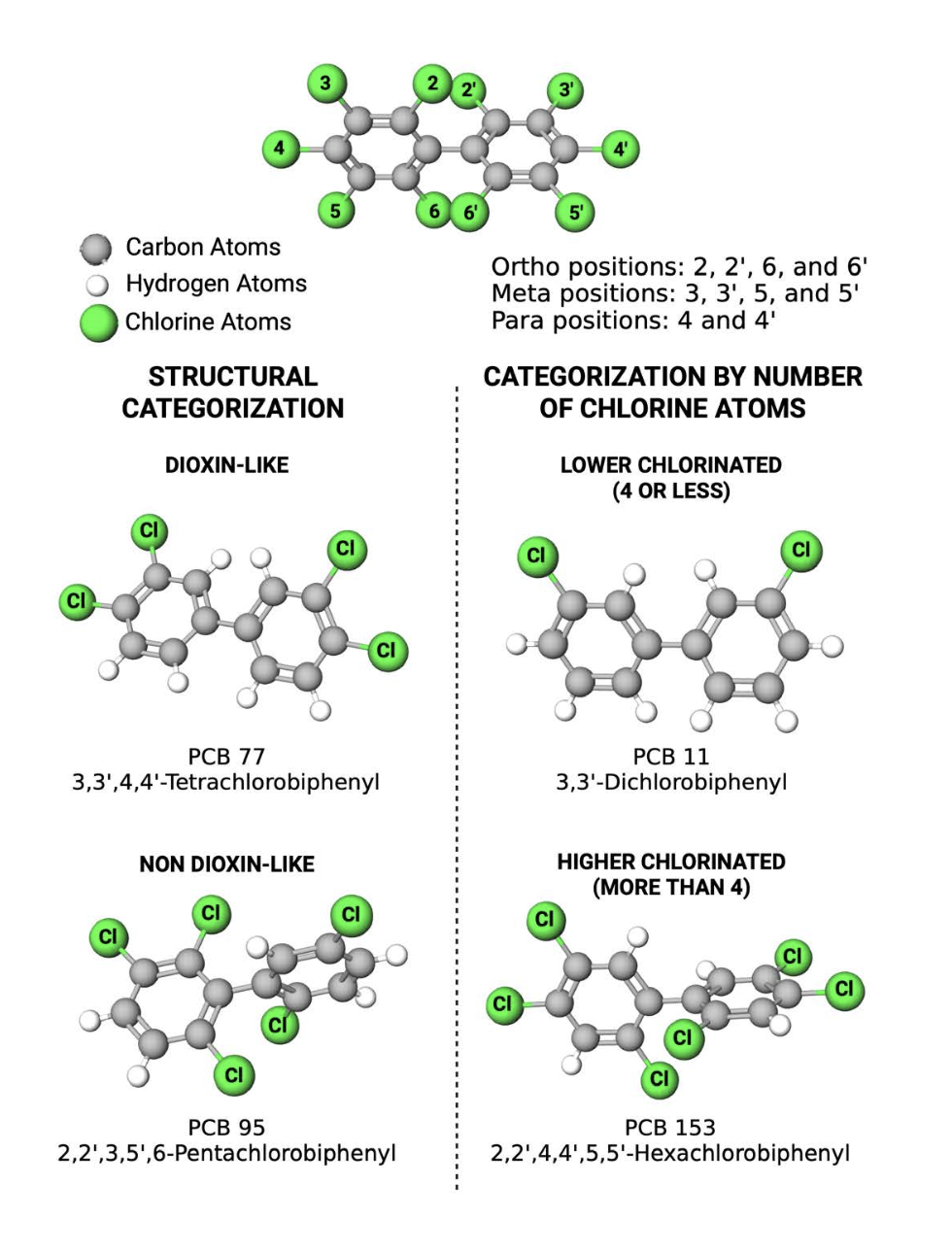
Chemically, PCBs are comprised of carbon, hydrogen, and chlorine atoms arranged as biphenyls (2 linked rings of 6 carbons each) with chlorine substituted for hydrogen at varying positions on the carbon rings (Figure 1). There are 209 possible PCB structures, which are individually referred to as congeners. Congeners vary with respect to their chlorine substitutions, the number and position of which influence the physical, chemical and toxicological properties of individual congeners.
Several schemes are used for categorizing PCBs (Figure 1). In one scheme, PCBs are broadly divided into dioxin-like (DL) or non-dioxin-like (NDL) congeners based on their structure. The DL PCB congeners typically have fewer than two chlorines at the ortho positions of the biphenyl and thus, like dioxin, tend to have a fairly rigid coplanar structure (both phenyl rings lie in the same plane). Of the 209 possible PCB congeners, approximately 12 are considered DL congeners. In contrast, the remaining PCBs, which are the NDL PCBs, have more than one ortho-substituted chlorine, and are typically non-coplanar (the phenyl rings are out of alignment relative to each other) with a more flexible structure.
Because of their structural differences, DL and NDL PCBs interact with different biological targets to elicit different profiles of toxicity. DL PCBs bind to and activate the aryl hydrocarbon receptor (AhR), which is the canonical “dioxin receptor”. Thus, like dioxin, DL PCB congeners are linked to increased risk of cancer, immune dysfunction and skin abnormalities. In contrast, NDL PCBs interact with receptors other than the AhR to interfere with normal brain development, alter immune and endocrine functions and promote metabolic disorders. As a result, the legacy PCB mixtures, which contained both DL and NDL PCBs, elicit complex toxicities.
Alternatively, PCB congeners can be categorized based on their number of chlorine substitutions. Congeners with four or less chlorine substituents are referred to as lower chlorinated PCBs (LC-PCBs); those with more than four chlorine substituents, as higher chlorinated PCBs (HC-PCBs). The number of chlorines influences volatility (ability of the congener to vaporize) such that increasing chlorination decreases volatility. Thus, the more volatile LC-PCBs comprise the majority of airborne PCBs found in major cities in the United States (U.S.) and in indoor air. The degree of chlorination also influences environmental persistence, with the HC-PCBs proving more resistant to environmental and metabolic degradation.
Environmental health hazards associated with polychlorinated biphenyls
The chemical stability that made PCBs so valuable commercially also resulted in their global environmental distribution. PCBs are resistant to environmental and metabolic degradation, with half-lives of individual congeners varying from days to years. PCBs are also highly lipophilic (they readily dissolve in fats), thus, they easily cross biological barriers, including the placenta and blood-brain barrier, and bioaccumulate in adipose and other fatty tissues. These dual properties of environmental persistence and lipophilicity, coupled with the widespread use and disposal of PCBs, enable these chemical contaminants to travel great distances via air, water and migratory species, to readily cycle between environmental compartments, and to biomagnify up food chains.
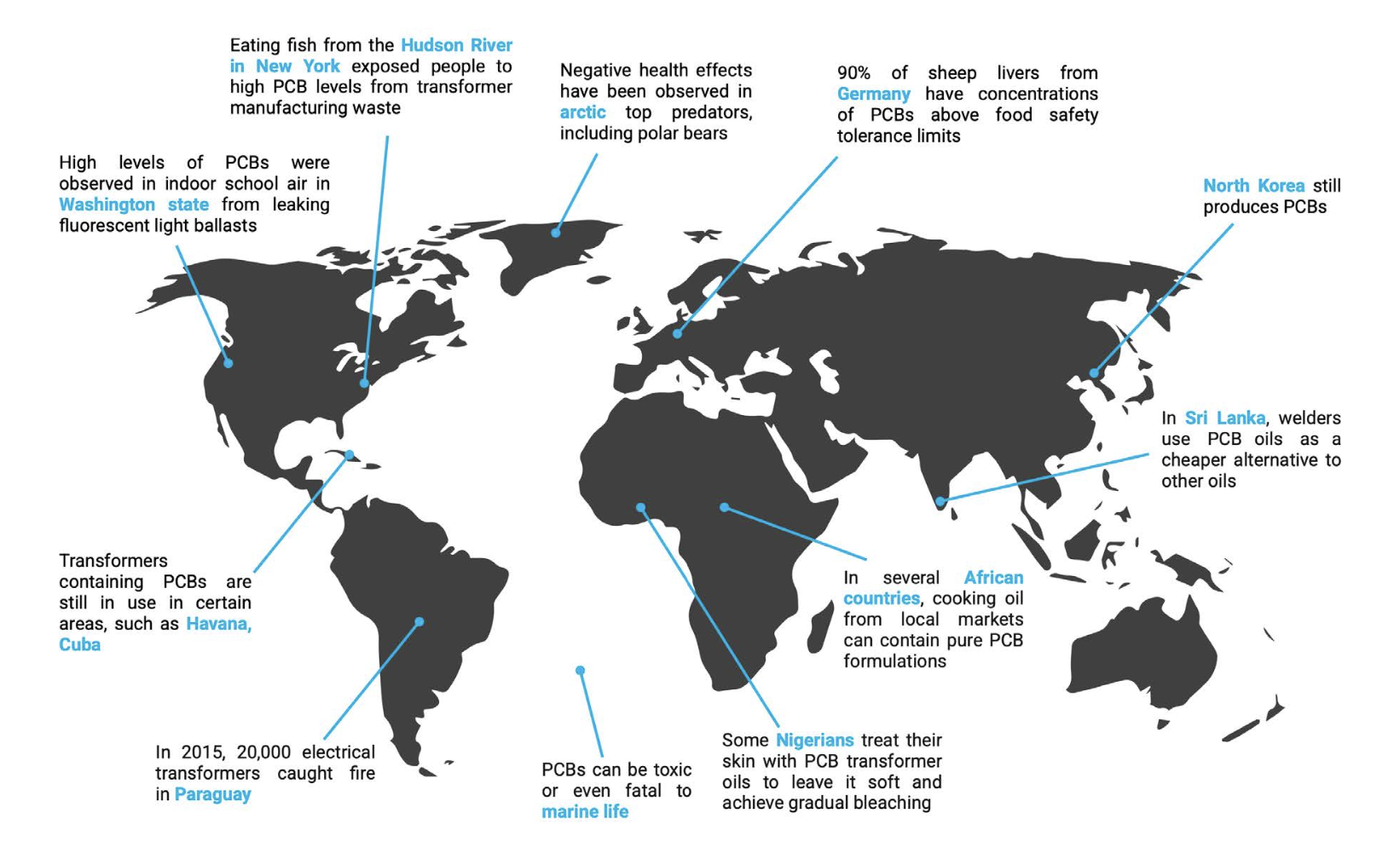
Current global monitoring efforts routinely detect PCBs in all environmental compartments, including human tissues, worldwide (Figure 2). The data are clear: all of us are exposed to PCBs and all of us carry detectable levels of PCBs in our tissues. However, the levels and types of PCB congeners vary within and across populations because of geographical and individual differences in exposure sources, routes of exposure, and differential degradation of individual congeners between individuals and over time within any one individual.
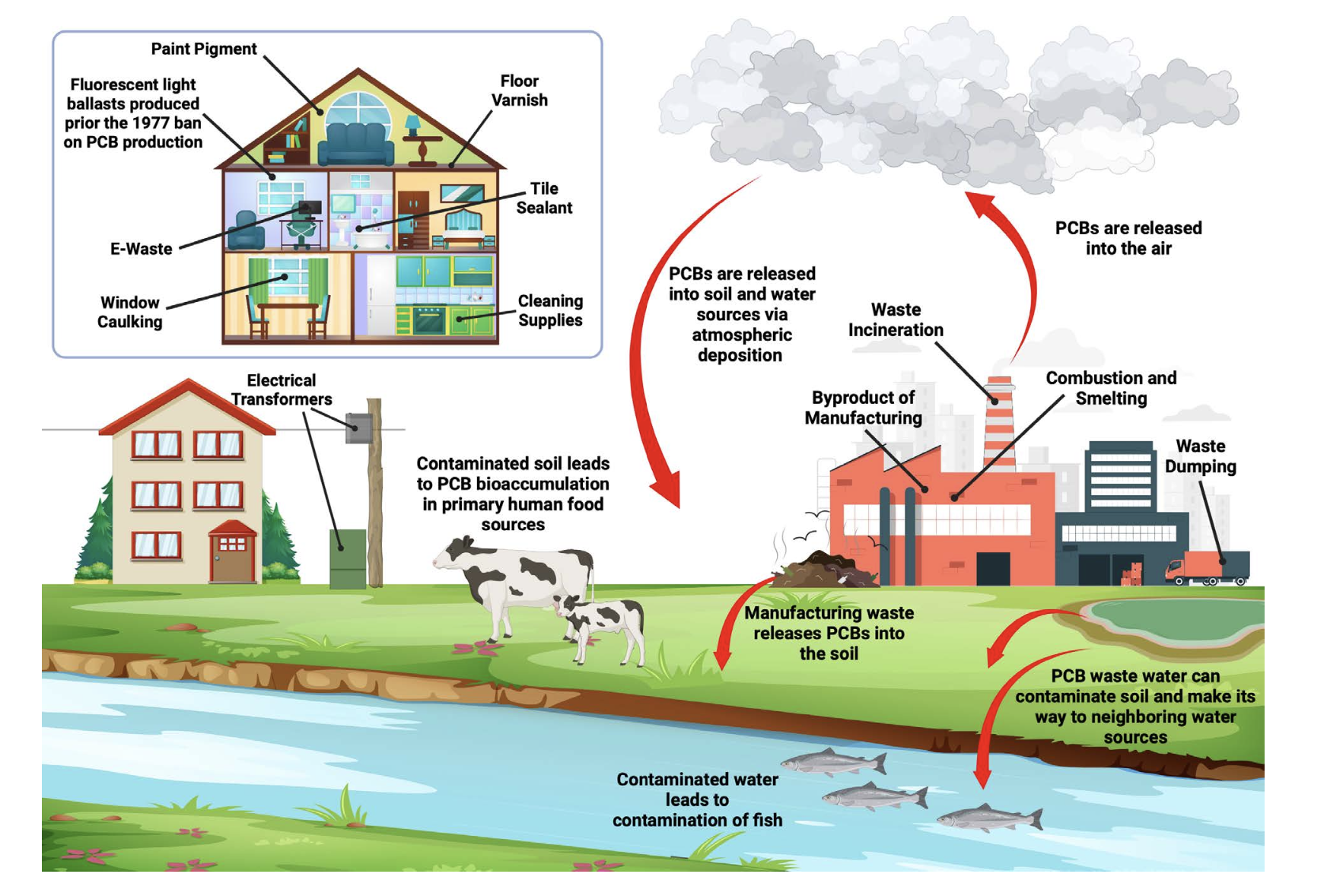
Sources of human exposure (Figure 3) include the continued release of PCBs in the legacy commercial mixtures from hazardous waste sites, PCB-containing equipment still in use, and degradation of construction materials and leaking of fluorescent light ballasts used in buildings erected prior to the ban on PCB production.
Additionally, contemporary PCB congeners that were not present in the legacy commercial mixtures are produced as inadvertent byproducts by various modern manufacturing processes, such as pigment production. PCBs are also formed and released unintentionally from several contemporary anthropogenic sources, including waste incinerators, cement kilns, metallurgical industry, residential combustion, e-waste, and diverse plastics.
Humans are exposed to PCBs throughout their lifetimes, including prenatally and during the early postnatal period through breastfeeding, with continuing exposure through multiple routes of exposure (Figure 4).
Occupational exposure historically occurred during the production or use of PCBs and PCB-containing products, and may still occur, for example, through the maintenance, repair, or recycling of old PCB-containing products or the disturbance of construction materials containing PCBs. Occupational routes of exposures include dermal and inhalation. The general population is typically exposed to PCBs via ingestion of contaminated food (especially fish from PCB- contaminated waters), and inhalation of contaminated air in both indoor and outdoor settings, especially at locations that still use electrical equipment and/or building and construction products containing PCBs.
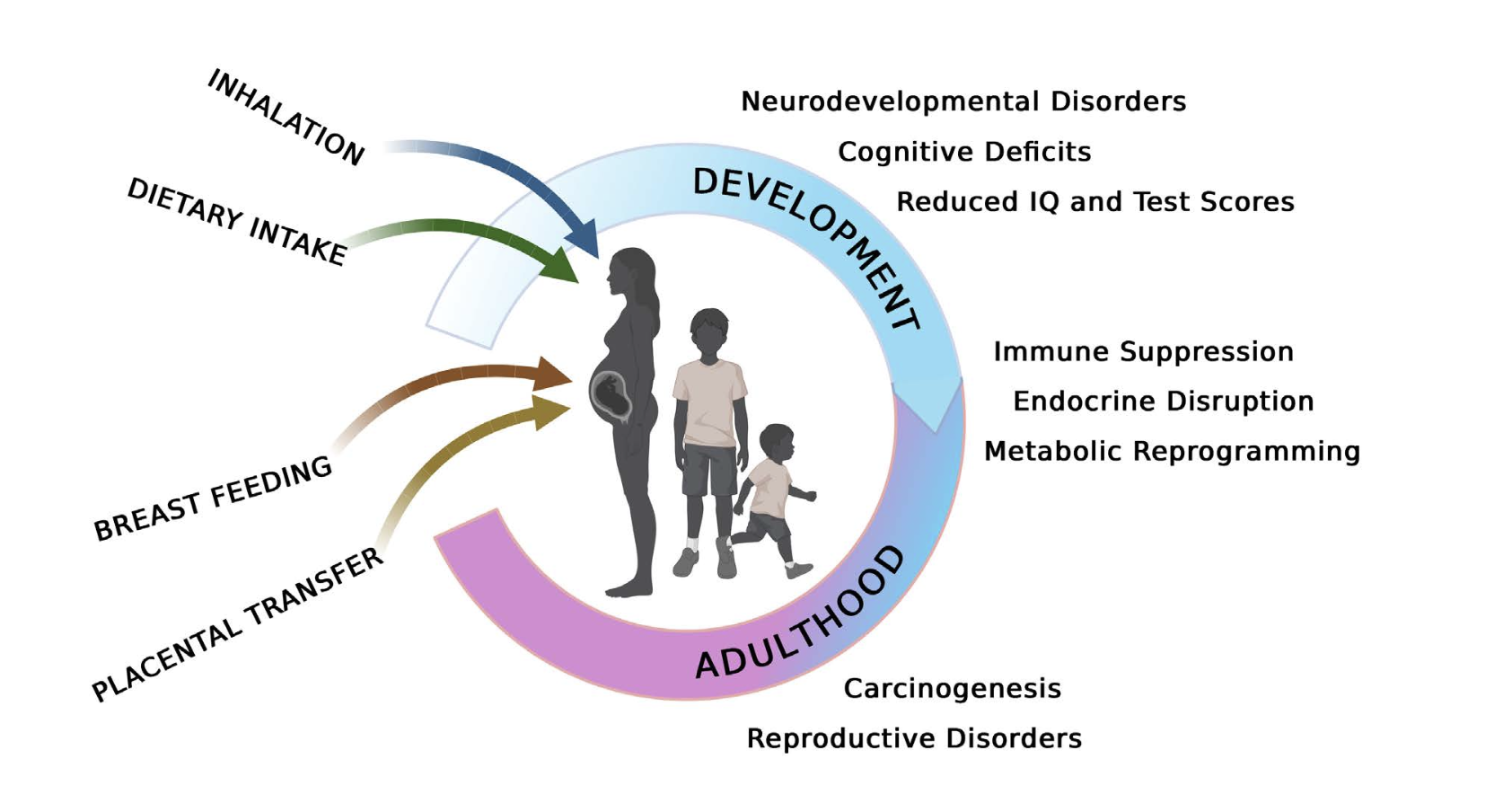
There is compelling evidence documenting the serious health effects of PCBs (Figure 4). The International Agency for Research on Cancer (IARC) classified PCBs as Group 1 “carcinogenic to humans”. Human epidemiological studies link occupational PCB exposures to rare liver cancers and malignant melanoma, and these human data have been corroborated by experimental studies in rodent models demonstrating that chronic administration of PCB mixtures and certain congeners resulted in the development of hepatic tumors. Subsequent mechanistic studies of PCB-induced liver tumors determined that PCBs have both cancer-initiating and promoting activities. Besides liver tumors, several PCB mixtures and congeners were reported to promote lung tumors in both mouse and rat models, which may be particularly relevant with inhalation exposures to PCBs.
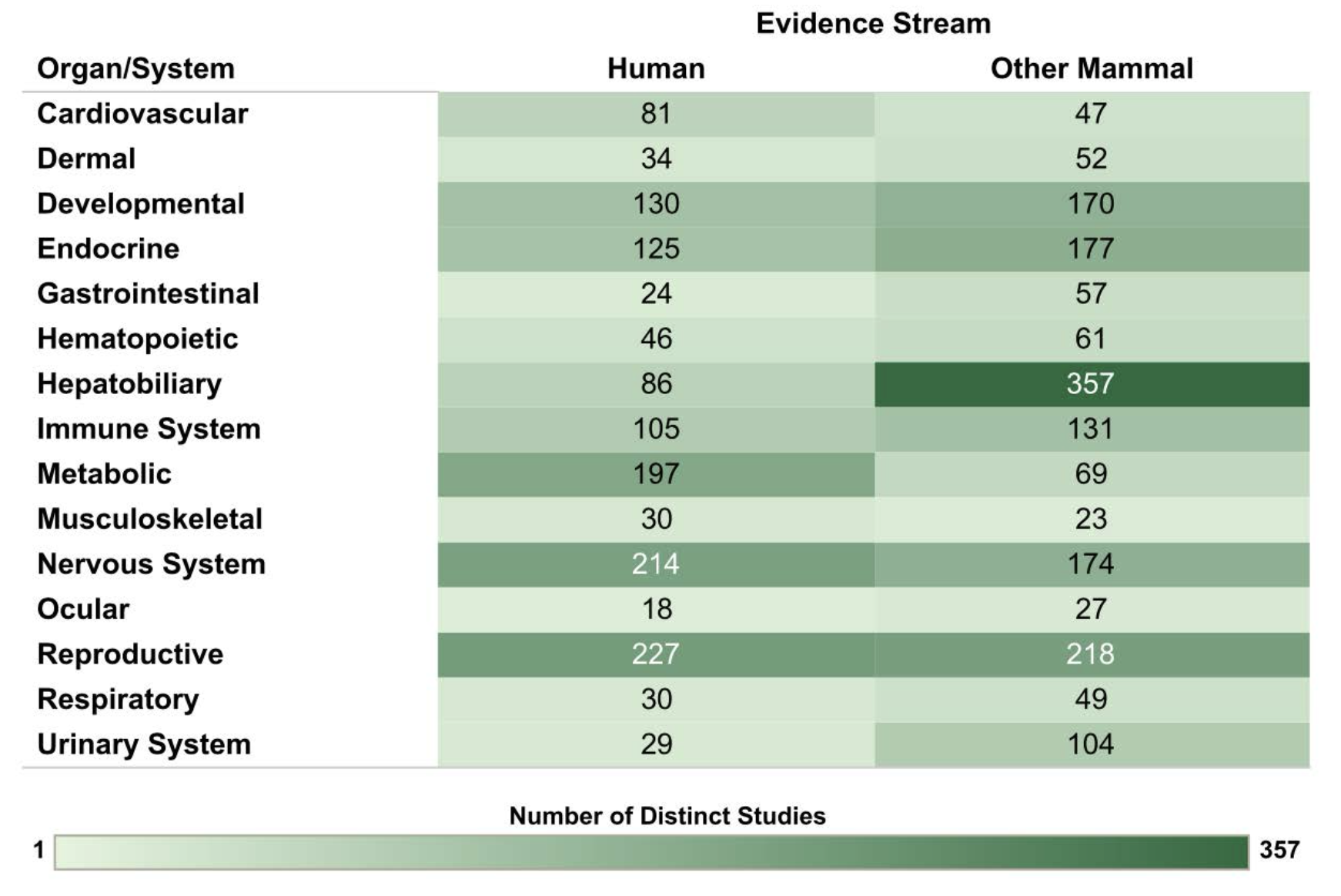
Strong associations between PCB exposure and numerous noncancer health endpoints have also been documented in both human epidemiological and experimental animal studies (Figures 4 and 5). Early studies of noncancer targets identified dermal, ocular, immune, endocrine and reproductive effects of PCBs. Toxic responses of dermal tissues to PCBs include dermal irritation and scar formation, and, specifically in the case of exposures to the DL PCBs, acne and chloracne. Other dermal endpoints evaluated in relation to PCB exposure include abnormal pigmentation, deformities in fingernails or toenails, hyperkeratosis, and gingival swelling or recession. Studies in mammals other than humans also identified alopecia (loss of hair), dermal/subcutaneous histopathological changes, and deficiencies in wound healing. With respect to immunological effects, PCBs primarily suppress immune function in humans, non-human primates and other mammalian species, including marine mammals. PCB-induced immunosuppression manifests as reduced antibody production in response to antigen challenge, decreased immune resistance to viral and other infections, and increased risk of non-Hodgkin’s lymphoma.
Endocrine effects of PCBs are most strongly linked to thyroid hormones, and include thyroid disease, decreased levels of circulating thyroid hormone, and thyroid gland histopathology. A strong database of human studies for thyroid function is available, comprising multiple moderate to large prospective studies assessing occupational and nonoccupational PCB exposures using valid endocrine biomarkers (i.e., thyroid hormone levels). The human studies are supported by extensive experimental data from nonhuman mammalian studies. Endocrine responses evaluated with PCB exposure also include differences in circulating adrenal hormone (stress hormones) concentrations and histopathological changes in the adrenal gland.
Reproductive effects of PCBs, which can be caused in part by PCB disruption of sex steroid production, distribution and/or signaling, have been documented in humans and experimental animal models. Studies of women who worked with PCBs in factories and human populations with heavy consumption of PCB- contaminated fish found decreased birth weight and a significant decrease in gestational age with increasing exposures to PCBs. In non-human primates, rats, mice and mink, PCB exposures were similarly observed to decrease birth weight, as well as reduce conception rates, live birth rates and sperm counts.
More recent studies indicate that PCBs can also disrupt metabolic and cardiovascular function. Metabolic endpoints that have been evaluated in relation to PCB exposures include impaired glucose metabolism and obesity. PCB effects on glucose metabolism were evaluated in humans based on the following endpoints: insulin resistance; impaired glucose tolerance/ prediabetes; type 2 diabetes mellitus; gestational diabetes; diabetes mellitus (not otherwise specified); and metabolic syndrome. Metabolic endpoints were also evaluated in a substantial number of nonhuman mammalian studies, including endpoints not represented in the human database, such as pancreas weight and histopathology, adipose tissue histopathology, and basal metabolic rate. Together, the human and animal databases support the potential for PCB-associated metabolic effects.
Human studies have also evaluated a range of pathogenically-related cardiovascular endpoints such as ischemic heart disease (also referred to as coronary artery disease or coronary heart disease), myocardial infarction (or heart attack), hypertension (high blood pressure), cerebrovascular disease, atherosclerosis (plaque buildup inside arteries and hardening and narrowing of their walls), and heart failure (inability to pump sufficient blood to organs and tissues). The results of these studies suggest a potential association between PCB exposure and atherosclerosis, especially in individuals prone to the development of vascular disease.
However, one of the most vulnerable targets of PCBs, particularly the NDL PCBs, is the developing nervous system (Figure 5). Human studies of nervous system endpoints were conducted in a range of age groups from infants to older adults. A recent review of the peer-reviewed literature identified seven domains that are most informative for assessing hazards of PCB exposure for the nervous system: cognitive function; attention, impulse control, externalizing behaviors, and internalizing behaviors (including activity level); executive function; social cognition and social behavior, including traits related to autism spectrum disorder (ASD); motor function/development; brain aging disorders; and auditory function. These domains are not mutually exclusive, as they arise in an interconnected brain. For example, executive function bears greatly on cognitive processes involved in planning and working memory and is often impaired among individuals with attention deficit hyperactivity disorder and ASD. In addition to the human studies, there is also an extensive database of studies that examined nonhuman mammalian behaviors that have face validity to the seven most informative domains described in humans. Together, the human and animal databases provide strong evidence indicating the potential for PCB- associated nervous system effects, especially for cognitive function, attention, impulse control, externalizing and internalizing behaviors, executive function, and motor function.
Environmental PCBs pose a risk not only to human health, but also the resilience of marine mammals, many of which are top predators and thus have very high levels of PCBs in their tissues. As indicated by Dr. Francesca Ginley from the Marine Conservation Society, PCBs are contributing to the collapse of the orca whale population in the U.K. An orca from the west coast of Scotland found dead in 2016 had PCB levels 100 time higher than the levels known to impact the health of marine animals. Research by the Zoological Society of London with the University of Glasgow last year revealed that U.K.-stranded orcas have 30 times over the toxic threshold for PCBs in their tissues. Such findings
have prompted a warning by environmental toxicologists that PCBs threaten to wipe out the orca population. Similar predictions have been made for other whale species, as well as various seal, porpoise, and otter populations worldwide.
The global ban on PCB production
Overwhelming evidence of the environmental persistence of PCBs, widespread human exposure, and links to both cancer and non-cancer health effects led various countries to ban PCB production in the late 1970s and early 1980s. These bans coalesced in an international ban on PCB production codified in the 2001 Stockholm Convention on persistent organic pollutants, or POPs, which obliges signatory countries to eliminate the use of PCBs in equipment by 2025 and to make determined efforts to lead to the environmentally sound management of waste liquids and equipment contaminated with PCB by 2028. To date, 185 countries have ratified the Convention.
The specific expectations set forth in the Stockholm Convention include:
- Parties to ban the production and new uses of PCBs;
- Parties to make determined efforts to identify, label and remove from use equipment (e.g., transformers, capacitors or other receptacles containing liquid stocks) containing PCBs by 2025;
- Parties to make determined efforts designed to lead to environmentally sound waste management of liquids containing PCB and equipment contaminated with PCB as soon as possible but no later than 2028;
- Parties to identify other articles containing PCB (open applications) and manage them in an environmentally sound manner; and
- Parties to allow export or import of PCBs only for the purpose of environmentally sound waste management.
The PCB situation today
The hope in 2001 was that the goals set forth in the Stockholm Convention would significantly decrease environmental levels of PCBs, which in turn would reduce the risk of adverse impacts of PCBs on the environment and human well-being. However, the reality in 2025 is that PCBs continue to pose a significant threat to humans and animals, and this risk may have actually increased in the past several decades. Several factors have contributed to this: (1) the failure of signatory nations to fulfil the expectations of the Stockholm Convention; (2) accelerated release of legacy PCBs from various sources; and (3) the production of PCBs not found in the legacy commercial PCB mixtures as byproducts in commercial chemical production.
As of 2025, progress in eliminating PCBs varies considerably across countries, with only 30% of the countries on track to reach the 2025 and 2028 goals. In January 2016, the United Nations estimated that only 3 million tonnes (17% of the total) of equipment and materials containing PCBs (transformers account for the largest share of the total mass) had been eliminated, at the rate of about 200,000 tonnes/year since 2000. That left 14 million tonnes (83% of the total) still to be eliminated. Addressing the remaining 83% would require the elimination of 1 million tonnes of PCB-containing oils and contaminated equipment per year to reach the 2028 target, and as of 2025 this target has not been met.
The quantities of PCBs (in use in equipment, stored, or eliminated) are estimated based on national reporting as well as answers to an online survey and a questionnaire. Information provided by countries reporting to the Basel Convention on PCB import and export (allowed for the purpose of environmentally safe waste management) are also taken into consideration. The “Report on progress towards the elimination of polychlorinated biphenyls”, in February 2019 (UNEP/ POPS/COP.9/INF/10) highlights the difficulties in estimating quantitative PCB data due to: (1) Incomplete national reporting (at the time the report was written, six months after the deadline for submission, only 59 Parties out of 182 had submitted their fourth national report); (2) limited coverage of voluntary answers to survey and questionnaire; (3) incomplete and inconsistent inventories: countries report differently on equipment containing PCBs in operation, equipment that has been decommissioned and stored waiting for proper final management, oil and other contaminated liquids, other contaminated materials, and on PCBs in open applications (not an obligation under the Stockholm Convention); (4) lack of analytical methods to identify waste containing PCBs; and (5) diverse interpretation of the meaning of “PCBs in use”.
There is well-documented evidence that inadequate management of known PCB sources increases human exposures. A study by scientists at Emory University in Atlanta, Georgia detected PCBs, PFAS and other chemical contaminants at the site of an old landfill, chemical manufacturing plants, and a dredged creek in Brunswick, Georgia in southeastern U.S. Blood tests from 100 longtime Brunswick residents, including former workers at the plants, show almost half of them have levels of PCB congeners found in Aroclor 1268, one of the legacy commercial PCB mixtures, that are far higher than U.S. averages. Interestingly, while higher than average levels of toxaphene, a toxic pesticide, was found in one-quarter of the study participants, levels of PFAS, mercury and other toxic metals, and other persistent organic pollutants in the study participants were within the ranges reported for the general population.
In addition to the ineffective management of equipment containing legacy PCBs and of PCB wastes, the release of legacy PCBs from PCB-containing equipment, construction materials, and landfills is accelerating as these sources age. Additionally, large amounts of PCBs that had been transported to and deposited in the polar ice caps, where they were effectively sequestered for decades, are now being re-released into the environment as glaciers melt due to global climate change. Adding to the environmental levels of PCBs are the production of “non-legacy” PCBs as inadvertent byproducts in commercial chemical syntheses, including the production of paints and pigments. However, recent research indicates the problem is broader than just paint and pigment production: chlorinated solvents that are used in chemical manufacturing are an even greater source of PCB production. This means that PCBs are present in small proportions in many of the chemical products being produced today, including high-volume production chemicals.
According to Dr. Dave Megson from Manchester Metropolitan University, when the volume of chemicals produced that contain PCB byproducts is taken into consideration, the small levels of PCBs within them add up to a massive number – about 45,000 tonnes per year in the U.S. alone. During peak commercial production in the 1970s, about 39,000 tonnes were made each year. On a global scale, current unintentional PCB production could be much higher. Compounding this problem, these “contemporary” PCBs are currently going undetected in many studies because the specific PCB congeners produced accidentally are different from the PCBs that were produced intentionally in the commercial mixtures 50 years ago.
Another contemporary source of PCBs is e-waste. Informal e-waste recycling poses substantial environmental and human health risks due to contamination by numerous chemical additives, including PCBs. A systematic review of research on polybrominated flame retardants (PBDEs), PCBs and organophosphate esters in e-waste recycling sites concluded that PCBs were a predominant contaminant. The study also found notable variations across different countries. For instance, high levels of PCBs were reported in China and India, which are major hubs for e-waste recycling. PCB concentrations in these regions often exceed international safety standards, posing severe risks for workers and local communities. Data from Africa and South America are sparse, despite the growing presence of informal e-waste recycling activities in these regions. While high concentrations
of PCBs are documented in environmental matrices collected within and near informal e-waste recycling sites, human biomonitoring and epidemiological studies are needed to determine whether this translates into higher risk of adverse health effects.
Of significant concern, the PCB congeners produced as inadvertent byproducts in commercial chemical synthetic processes are being widely detected in the environment and in human tissues, including breastmilk, placenta, and the fetal brain. In a recent study of PCBs in post-mortem human brain samples, these non-legacy PCB congeners were detected at significantly higher levels than legacy PCBs in prenatal and postnatal brain tissue. Research in experimental models demonstrate that at least some of these non-legacy PCBs can interfere with normal brain development to cause behavioral phenotypes characteristic of neurodevelopmental disorders, such as autism and attention-deficit disorder, as well as immune and endocrine dysfunction.
How do we mitigate risks associated with PCB exposures?
It is clear that PCBs are ubiquitous in our environment, and that environmental levels of these forever chemicals are not decreasing, and in some geographic locations, may actually be increasing. It is also now obvious that monitoring efforts focused on the PCB congeners found in the legacy commercial PCB mixtures provide inadequate information about contemporary human exposures. To move forward, it will be necessary to develop more holistic approaches to PCB monitoring efforts not only to get a clearer picture of global progress towards achieving the goals of the Stockholm Convention, but also to understand the exposure potential to contemporary PCBs produced as inadvertent byproducts of modern chemical production processes.
Tackling the PCB problem will require international standardization of methods and approaches for detecting and reporting environmental PCB levels. Since PCBs readily cross national borders, international harmonization of practices and policies for managing PCB-containing equipment and wastes will be required to realize any real mitigation. Research focused on reducing or eliminating the inadvertent production of PCB byproducts in the chemical industry is desperately needed. So too is epidemiological and toxicological research to better understand the health risks associated with the non-legacy PCBs. Helping people understand the risks of PCBs is key to making changes. When consumers demand better solutions, companies and policymakers listen.
While meaningfully addressing the PCB problem will require a significant investment of resources, the investment is likely to result in significant benefits given the overwhelming evidence that continued release of PCBs into the environment is worsening the health and well-being of the environment and humans not only now but for generations to come.


