Cambridge‑led PIONEER trial finds that a progesterone‑mimicking drug used for hot flushes boosts anti‑oestrogen therapy and slows tumour growth in early breast cancer
A new Cambridge‑led clinical trial has found that a drug traditionally used to treat hot flushes can enhance the effectiveness of standard anti‑oestrogen treatment in women with early breast cancer. Building on this finding, the PIONEER study demonstrated that adding a low dose of a progesterone‑mimicking drug slowed tumour growth, suggesting a promising strategy to improve outcomes for patients on long‑term hormone therapy.
How megestrol acetate could change breast cancer care
Megestrol acetate has proven to help patients manage hot flushes associated with anti-oestrogen breast cancer therapies, and so could help them continue taking their treatment. However, the PIONEER trial has shown that the addition of low-dose megestrol to such treatment may also have a direct anti-cancer effect.
About three-quarters of breast cancers are ER-positive, meaning the tumours have many oestrogen receptors. These patients receive anti-oestrogen medication, which lowers oestrogen levels but can cause menopause-like symptoms such as hot flushes and muscle pain.
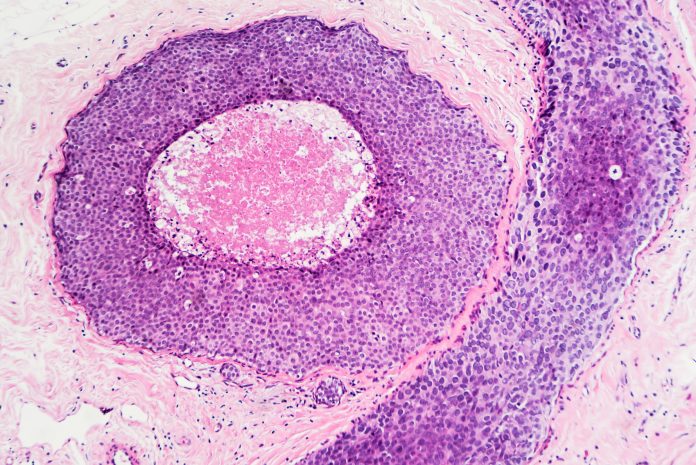
Some ER-positive breast cancer patients have high levels of progesterone receptor (PR) and typically respond better to the anti-oestrogen hormone therapy. To explain why, Professor Jason Carroll and colleagues at the Cancer Research UK Cambridge Institute used cell cultures and mouse models to show that the hormone progesterone prevents ER-positive cancer cells from dividing by indirectly blocking ER signalling. This results in slower tumour growth.
Professor Carroll, who co-leads the Precision Breast Cancer Institute and is a Fellow of Clare College, Cambridge, said: “These were very promising lab-based results, but we needed to show that this was also the case in patients. There’s been concern that taking hormone replacement therapy – which primarily consists of oestrogen and synthetic versions of progesterone (called progestins) – might encourage tumour growth. Although we no longer think this is the case, there’s still been residual concern around the use of progesterone and progestins in breast cancer.”
Inside the PIONEER trial: Testing megestrol’s impact
To build on these laboratory findings and understand whether targeting the progesterone receptor in combination with an anti-oestrogen could slow tumour growth in patients, the researchers developed the PIONEER trial.
In the PIONEER trial, a total of 198 patients were recruited at ten UK hospitals and randomised into one of three groups: one group received only letrozole; one group received letrozole alongside 40mg of megestrol daily; and the third group received letrozole plus a much higher daily dose of megestrol, 160mg. The treatment was administered for two weeks prior to surgery to remove the tumour. The percentage of actively growing tumour cells was assessed at the start of the trial and then again before surgery.
The researchers found that adding megestrol enhanced letrozole’s ability to block tumour growth, with comparable effects at both 40mg and 160mg.
Joint first author Dr Rebecca Burrell from the Cancer Research UK Cambridge Institute and CUH said: “In the two-week window that we looked at, adding a progestin made the anti-oestrogen treatment more effective at slowing tumour growth. What was particularly pleasing to see was that even the lower dose had the desired effect.
“Although the higher dose of progesterone is licensed as an anti-cancer treatment, over the long term, it can have side effects, including weight gain and high blood pressure. But just a quarter of the dose was as effective, and this would come with fewer side effects. We know from previous trials that a low dose of progesterone is effective at treating hot flushes for patients on anti-oestrogen therapy. This could reduce the likelihood of patients stopping their medication, and so help improve breast cancer outcomes. Megestrol – the drug we used – is off-patent, making it a cost-effective option.”