UCL and UCLH launch the Win‑Glio trial led by Dr Paul Mulholland, offering ipilimumab immunotherapy to newly diagnosed glioblastoma patients ahead of standard treatment
In a groundbreaking move to tackle one of the deadliest forms of brain cancer, researchers at UCL and UCLH have launched a pioneering clinical trial testing immunotherapy as a first-line treatment for glioblastoma. The trial, known as Win‑Glio, offers new hope for patients facing a diagnosis that has long been considered incurable.
Advancing brain cancer research
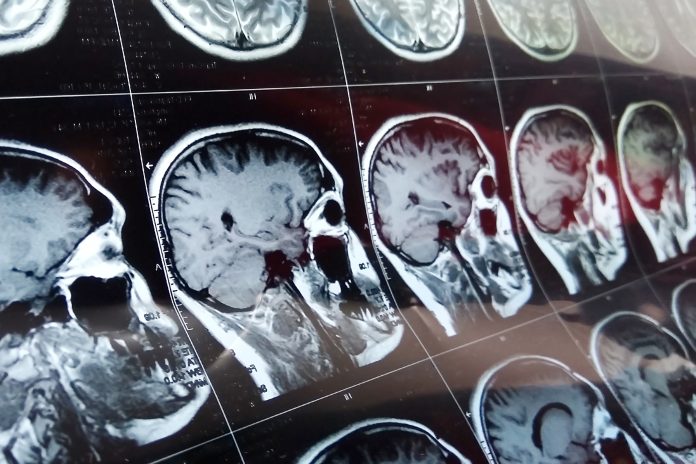
Glioblastoma is a fast-growing type of brain tumour and is the most common type of malignant brain tumour in adults. The main treatments are surgery, radiotherapy, and chemotherapy.
In this new trial, patients will receive an immunotherapy treatment using the drug ipilimumab, before standard treatment when their immune system is at its strongest. Patients will undergo standard treatments and receive ongoing monitoring.
The trial follows the NeAT-GLIO clinical trial, led by Dr Mulholland and funded by the Jon Moulton Charity Trust, which had to close due to a lack of patient recruitment. However, the one patient recruited to that trial, 43-year-old Ben Trotman, is doing well, with no active tumour present on scans more than two and a half years after treatment.
Dame Siobhain McDonagh MP led a fundraising campaign that raised more than £1 million to cover the costs of the new trial, including holding a dinner to celebrate the legacy of her sister, Baroness Margaret McDonagh. She has been on a mission to find a cure for glioblastoma since losing Margaret to the disease in 2023, a campaign started by Margaret herself after her diagnosis.
Dame Siobhain, MP for Mitcham and Morden and chair of the all-party parliamentary group on brain tumours, said: “My beloved sister Margaret was appalled to discover that there had been no advances in brain cancer treatment for decades when she was diagnosed with glioblastoma.
“Changing this was Margaret’s final campaign and one that I have continued in her memory. I am so grateful to the many people who knew and respected Margaret, who have come together and helped to raise funds and campaign for this new trial that we are calling Margaret’s Trial.”
Dr Mulholland said: “When I met Margaret, she said to me, ‘What can I do to support you to cure this disease?’. I am incredibly grateful to her and to Siobhain, whose campaigning and fundraising in her sister’s memory have led to this new clinical trial opening for patients with this most aggressive form of brain cancer that has such a poor prognosis, with most patients surviving just nine months after diagnosis.
“The crucial element of this trial is that patients will have their immune system boosted by the drug before they have any other treatment, when they are fit and well enough to tolerate the immunotherapy.
“We saw with Ben, the one patient recruited to the NeAT-GLIO immunotherapy study, that he has had clear scans since having the treatment and the tumour hasn’t returned more than two and a half years later.”
“We’re taking everything we have learned from previous trials into this new study, and we are already planning follow-on trials. I aim to find a cure for glioblastoma, and I am very thankful to Dame Siobhain McDonagh MP, the Jon Moulton Charity Trust and The National Brain Appeal for their support on this journey.”
Improving outcomes for patients with glioblastoma
The National Brain Appeal is funding two positions to support Dr. Mulholland’s research: a clinical nurse specialist for glioblastoma patients at diagnosis and a senior computational biologist to analyse genetic data and design computational pipelines for research.
Dr Mulholland added: “We aim to rapidly bring about improved outcomes for patients with glioblastoma by bringing together the best science, with a multidisciplinary team of clinicians, experimental scientists, bioinformaticians and the pharmaceutical industry. We want to find a cure for this devastating disease.”