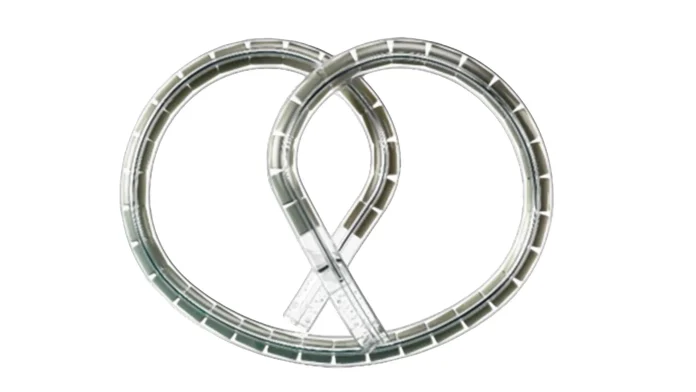
The TAR‑200 slow‑release bladder implant cleared tumours in 82% of patients with high‑risk non‑muscle‑invasive bladder cancer, offering a promising new treatment approach
A small implant called TAR‑200 has shown remarkable results in patients with high‑risk bladder cancer, eliminating tumours in 82 % of participants. Inserted via catheter, the device delivers chemotherapy gradually over several weeks, providing a major advancement for patients with limited treatment options.
“Traditionally, these patients have had minimal treatment options. This new therapy is the most effective one reported to date for the most common form of bladder cancer,” said Sia Daneshmand, MD, director of urologic oncology at Keck Medicine of USC and lead author of the study, which was published in the Journal of Clinical Oncology. The findings of the clinical trial represent a breakthrough in the treatment of certain types of bladder cancer, leading to improved outcomes and potentially saving lives.
TAR-200: A chemotherapy delivery system
The TAR-200 device is a pretzel-shaped implant that holds the chemotherapy drug gemcitabine. It is inserted into the bladder using a catheter and gradually releases the drug over a three-week cycle.
Typically, gemcitabine is administered as a liquid solution that remains in the bladder for only a few hours, which often limits its effectiveness in killing cancer cells.
“The theory behind this study was that the longer the medicine sits inside the bladder, the more deeply it would penetrate the bladder and the more cancer it would destroy,” Daneshmand explained. “And it appears that having the chemotherapy released slowly over weeks rather than in just a few hours is a much more effective approach.”
A worldwide study across 144 sites
The study took place at 144 sites worldwide, including Keck Hospital of USC. It enrolled 85 patients diagnosed with high-risk non-muscle-invasive bladder cancer.
Patients in the trial had previously received treatment with Bacillus Calmette-Guérin (BCG), an immunotherapy drug that is currently the standard of care. However, for a portion of patients, BCG is ineffective and the cancer returns.
“The standard treatment plan for these patients was surgery to remove the bladder and surrounding tissue and organs, which has many health risks and may negatively impact patients’ quality of life,” said Daneshmand.
The doctors administered TAR-200 every three weeks for six months, followed by four treatments per year for the next two years. Out of 85 patients, 70 experienced complete tumour disappearance, and nearly half remained cancer-free after one year. The therapy was well tolerated, with minimal side effects reported.
Researchers found that combining TAR-2000 with another immunotherapy drug was less effective and caused more side effects than TAR-200 alone.
The study will continue to be monitored for another year.
Exploring slow-release drug delivery systems
The study forms part of the research drive to explore slow-release drug delivery systems for cancer treatment. These approaches aim to provide long-lasting exposure to cancer-fighting drugs directly at the tumour site.
“We are at an exciting moment in history,” said Daneshmand, who has been investigating this technology since 2016. “Our mission is to deliver cancer-fighting medications into the bladder that will offer lasting remission from cancer, and it looks like we are well on our way toward that goal.”
The U.S. Food and Drug Administration has granted TAR-200 a New Drug Application Priority Review, which allows the agency to expedite its evaluation.