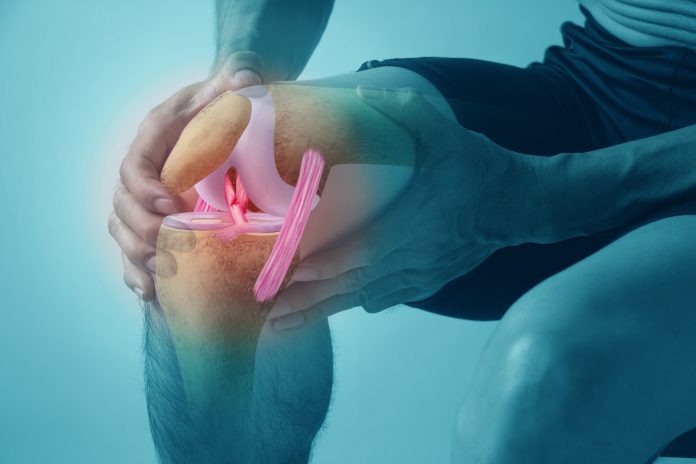
Stanford researchers develop a therapy that regenerates cartilage and blocks osteoarthritis in ageing joints, showing promise for future treatments that restore joint health
Stanford researchers have developed a new therapy that regenerates cartilage by targeting a key protein involved in ageing. In preclinical studies, the treatment restored healthy cartilage in ageing mice and significantly reduced joint degeneration, offering hope for therapies that could improve osteoarthritis and joint health and reduce the need for hip and knee replacements.
The research was published in Science.
Targeting the root cause of osteoarthritis
Osteoarthritis is a degenerative joint disease, and there are currently no approved drugs capable of slowing or reversing the underlying cartilage damage. Most existing treatments manage pain and inflammation rather than the root cause. The Stanford approach directly targets cartilage degeneration, marking a major shift in treatment strategy.
The protein at the centre of the research is 15-PGDH, also called a gerozyme, an enzyme that speeds up chemical reactions in the body. The amount of this enzyme increases with age and was first identified by the same Stanford team in 2023 as a driver of tissue decline in older organisms.
In mice, higher levels of 15-PGDH are linked to declining muscle strength with age. Blocking the enzyme using a small molecule boosted muscle mass and endurance in older animals. Forcing young mice to produce excess 15-PGDH caused muscle weakening and shrinkage.
A new mechanism for cartilage regeneration
In most tissues, repair occurs through the activation and specialisation of stem cells, which can develop into many different cell types. Cartilage, however, appears to regenerate differently. Instead of relying on stem cells, existing cartilage cells, called chondrocytes, which build and maintain cartilage, shift their gene behaviour to behave more like younger cells.
“This is a new way of regenerating adult tissue, and it has significant clinical promise for treating arthritis due to ageing or injury,” said Helen Blau, PhD, professor of microbiology and immunology at Stanford. “We were looking for stem cells, but they are clearly not involved. It’s very exciting.”
Researchers prevent osteoarthritis after joint injury
Osteoarthritis develops when joints are stressed by ageing, injury, or obesity. Chondrocytes (cartilage cells) begin releasing molecules that trigger inflammation and start breaking down collagen, the main protein that gives cartilage its structure. As collagen is lost, cartilage becomes thinner and softer, leading to inflammation, swelling, and pain.
Earlier studies from Blau’s lab found that prostaglandin E2, a molecule important for tissue healing, is essential for tissue repair, while the enzyme 15-PGDH breaks it down. Blocking 15-PGDH or increasing prostaglandin E2 previously promoted tissue repair in young mice.
Building on this work, researchers found that 15-PGDH levels double with age in knee cartilage. Treating older mice with a 15-PGDH inhibitor, either systemically or by knee injection, regenerated age-damaged cartilage across the joint. Newly formed tissue was healthy hyaline cartilage, presenting strong evidence for reversal of age-related cartilage degeneration.
Mice received twice-weekly injections of the inhibitor for four weeks following joint injury. These mice were far less likely to develop osteoarthritis. In contrast, untreated animals showed doubled 15-PGDH levels and developed osteoarthritis within four weeks.
“Prostaglandin E2 has often been associated with inflammation and pain,” Blau said. “But this research shows that, at normal biological levels, modest increases can actually promote regeneration.”
Further analysis revealed that chondrocytes in older mice expressed more genes associated with inflammation and cartilage-to-bone conversion, and fewer genes involved in cartilage formation. Treatment reversed these patterns.
One group of chondrocytes, producing 15-PGDH and cartilage-degrading genes, decreased from 8% to 3%. Another group associated with fibrocartilage formation declined from 16% to 8%. Meanwhile, a third population, cells that did not produce 15-PGDH and instead expressed genes responsible for hyaline cartilage formation and extracellular matrix maintenance, rose from 22% to 42%.
Together, these changes reveal that treatment restores an overall youthful cartilage profile independently of stem or progenitor cells, reinforcing the therapy’s distinct mechanism.
Evidence from Human Cartilage Samples
The researchers also tested cartilage taken from patients undergoing total knee replacement surgery for osteoarthritis. After one week of treatment with the 15-PGDH inhibitor, the tissue showed fewer chondrocytes (cartilage cells) producing 15-PGDH, reduced genetic signals for breaking down cartilage and for fibrocartilage, and early signs that the tissue was starting to regenerate healthy articular cartilage (the smooth cartilage that covers bone ends in joints).