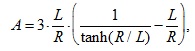Zhihui Wang1, Subrata Sen2, and Vittorio Cristini1
1Center for Precision Biomedicine, Brown Foundation Institute of Molecular Medicine, University of Texas Health Science Center at Houston (UTHealth) McGovern Medical School, Houston, TX 77030, USA
2Department of Radiation Oncology, University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA
Ductal carcinoma in situ (DCIS) is an early form of breast cancer where the tumor originates and is confined to the breast duct. While DCIS is not a fatal disease, it often progresses to invasive ductal carcinoma (IDC), which can be fatal. To combat DCIS, women may elect to have the entire breast removed or have breast conservation surgery, in which the portion on the breast that is identified by mammography to be affected by DCIS is removed. However, 20–50% of breast conservation cases require an additional surgery to remove all DCIS, because the “surgical volume” is poorly defined by mammography.
We have been investigating a combined mathematical modeling and pathology data analysis approach to predict outcome for breast cancer prior to actual treatment. One of our major goals is to accurately describe how DCIS develops to aid in therapeutic planning in order to define the volume of breast tissue which should be surgically removed, i.e., the surgical volume. An accurate assessment of this quantity is instrumental in providing physicians all necessary information before surgery. In our recent modeling work on DCIS (Edgerton et al., Anal Cell Pathol 2011, PMC3613121), we sought to determine if an accurate prediction of tumor size is achievable through measurements that are taken from a single biopsy.
This model is composed of a system of partial differential equations (PDEs; see the original article) and has three key parameters, which can all be assessed in pathological analysis of patient biopsies performed at a single time point in between detection through mammography and surgical planning. The three key parameters are related to tumor development, involve cell proliferation and death, as well as the physical ability of cells to receive nutrients depending on the diffusion properties of the microenvironment. For clinical translation of the model, we further derive the following analytical solution:
where A is the patient-specific ratio of cell apoptosis to proliferation rates averaged over the multitude of ducts within the surgical volume, L the diffusion penetration distance of nutrients, and R the geometric-mean tumor surgical radius. This equation can be calibrated from the results of immunohistochemistry (IHC), which determines cell proliferation and death. IHC involves staining the cells in the proliferative and apoptotic state, and provides the measurements that drive the proliferative and apoptotic index values. When L and A are known, determining the value of R is simply a matter of mathematics. In this way, this equation uses the cell-scale values for calibrating the tissue-scale continuum model predicting surgical volume.
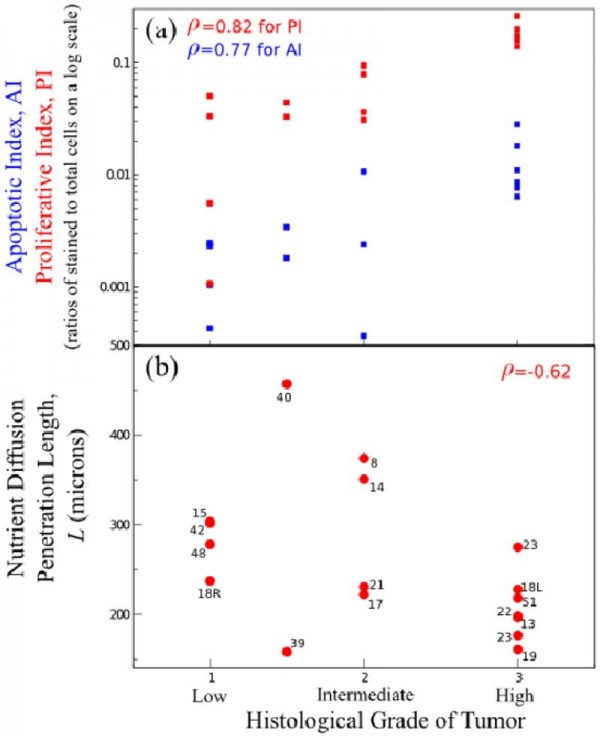
We examined 17 excised DCIS tumors, and found that mammography overestimated the tumor size in ten cases and underestimated the tumor size in seven cases. This result indicates that the correlation between mammography, nuclear grade, and the final observed tumor size is poor. We also found that the correlation between the sizes observed after surgery and those predicted by the model were close (see Fig. 1). The apoptotic and proliferative indices show similar trends in their relationships with tumor grade (Fig. 1a). This confirms that net proliferation (i.e., ratio of PI to AI), and thus tumor size, has a weak correlation with histologic grade. To clarify, the histologic grade of a tumor was assigned by a pathologist with low, low/intermediate, intermediate, or high grade; the lower the grade, the more the more slowly the cancer cells grow, and the better the patient prognosis. Moreover, viable-rim thickness of tumor in ducts, and thus L, decreases as a function of histological grade (Fig. 1b). It can be interpreted that more proliferative, high-grade tumors result in tightly packed cellular structures, and thus are likely to hamper oxygen and nutrient diffusion. Lack of oxygen in high-grade tumors would drive hypoxia-inducible factors and cell migration, which may lead to penetration of the ductal wall and infiltration of the tumor into the breast stroma.
Figure 1. Correlations of IHC and morphometric measurements with nuclear grade. (a) Average apoptotic (AI) and proliferative (PI) indices for each tumor as increasing functions of nuclear grade. Observed correlations ρ = 0.82 for PI and ρ = 0.77 for AI indicate that both indices have similar correlations with tumor grade. (b) Nutrient diffusion penetration length L from average measured viable-rim thickness in each tumor’s ducts is a decreasing function of nuclear grade. Reproduced with permission from Edgerton et al., Anal Cell Pathol 34(5):247-63.
In summary, through integrated mathematical modeling, it is possible to describe where a tumor is in its development, and, for DCIS, where the edge of the tumor is located, which aids in surgical planning. We designed our approach so that all data being supplied to the model could be derived from a patient’s biopsy and integrated into the clinical workflow. The practice will lead to less subjective analysis of tumors and an individualized approach to surgical removal of DCIS. In this context, we also propose that computer simulations of tissue scale parameter perturbations in progressively better elucidated genetic alterations in epithelial and stromal component of tumors will help develop more accurate models predictive of DCIS tumor volumes.
Contact information:
Vittorio Cristini, Ph.D.
University of Texas Health Science Center at Houston (UTHealth) McGovern Medical School
Center for Advanced Biomedical Imaging (CABI) building, Room 3SCR6.4644
1881 East Road, Houston, TX 77054, USA
Website: https://www.uth.edu/imm/profile.htm?id=228325bf-1821-41b9-a0b8-37ca77d47c88
ISI Highly-Cited Researchers in Mathematics: http://highlycited.com
Google scholar: http://scholar.google.com/citations?user=uwl5tw0AAAAJ&hl=en&oi=ao
Phone: +1 713-486-2315
Fax: +1 713-796-9697
E-mail: Vittorio.Cristini@uth.tmc.edu
Additional resources:
http://www.internationalinnovation.com/taking-cancer-out-of-the-equation/
http://www.pnas.org/content/110/35/14266.long
http://www.hindawi.com/journals/acp/2011/803816/abs/
http://journals.plos.org/ploscompbiol/article?id=10.1371/journal.pcbi.1004969