Researchers discover how chemokine CXCL12 triggers red blood cell maturation, paving the way for efficient artificial blood production
In a significant leap toward artificial blood, scientists at the University of Konstanz and Queen Mary University of London have uncovered how a single molecule, chemokine CXCL12, triggers a crucial step in red blood cell development. This discovery solves a decades-old mystery surrounding the expulsion of nuclei in red blood cell precursors, a vital process unique to mammals.
The findings, published in Science Signaling, could dramatically improve the efficiency of artificial blood production, opening new avenues for treating rare blood disorders and managing donor shortages worldwide.
Scientific breakthrough
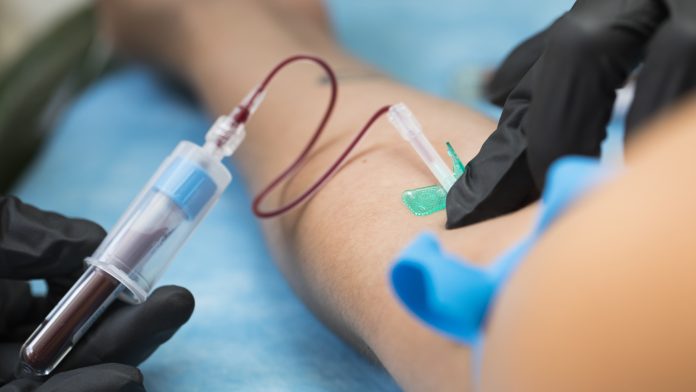
Natural blood production occurs in the bone marrow, where stem cells develop into erythroblasts, precursor cells that mature into erythrocytes (red blood cells). “In the final stage of an erythroblast’s development into an erythrocyte, the erythroblast expels its nucleus. This process only occurs in mammals, allowing them to make more room for haemoglobin involved in the transport of oxygen”, Doctor Julia Gutjahr, a biologist at the Institute of Cellular Biology and Immunology Thurgau at the University of Konstanz, explained.
Now that the process of stem cell maturation into erythrocytes is nearly optimized, it was previously unclear what factors induced the expulsion of the nucleus. “We discovered that the chemokine CXCL12, found mainly in bone marrow, can trigger such nucleus expulsion, albeit in an interplay with several factors. By adding CXCL12 to erythroblasts at the right moment, we were able to induce the expulsion of their nucleus artificially,” continued Gutjahr.
This discovery represents a scientific breakthrough and could significantly enhance the efficiency of artificial blood production; however, further research will be necessary.
“We are currently investigating how to use CXCL12 to optimize the artificial production of human erythrocytes,” Gutjahr explained. “Importantly, apart from immediate practical application for the industrial production of red blood cells, our results brought a completely new understanding of cell biological mechanisms involved in erythroblast responses to chemokines. While all other cells migrate when stimulated by CXCL12, in erythroblasts this signalling molecule is transported into the interior of the cell, even into the nucleus”, adds Rot. “There, it accelerates their maturation and helps to expel the nucleus. Our research shows for the first time that chemokine receptors not only act on the cell surface but also inside the cell, thus opening entirely new perspectives on their role in cell biology,” Professor Antal Rot commented.
Reprogramming cells and generating artificial blood production
Stem cells are currently the most effective method for producing artificial blood, with nuclear expulsion occurring in approximately 80% of cells. However, stem cell sources are limited, relying on the isolation of stem cells from umbilical cord blood or bone marrow donations for the treatment of specific diseases, which is not feasible for the mass production of blood to fulfil clinical needs.
It has recently become possible to reprogram different types of cells into stem cells and use them to generate red blood cells. This approach offers an almost unlimited cell source for artificial blood production, but takes much longer, and the success rate for nucleus expulsion is only about 40%. “Based on our new findings highlighting the key role of CXCL12 in triggering nuclear expulsion, we can expect that using CXCL12 should bring significant improvement in producing red blood cells from reprogrammed cells,” said Gutjahr.
If large-scale production becomes possible, a wide range of applications could emerge. “Even though body cells are readily available, the lab-based production process will remain complex. But it would enable the targeted generation of rare blood types, help bridge shortages, or allow individuals to reproduce their blood for specialized treatments in many different diseases,” concluded Gutjahr.