A new class of nanotherapy blockades the genetic drive for multiple myeloma progression. Gregory M. Lanza and Michael H Tomasson, of Department of Medicine, Washington University School of Medicine explain…
Multiple myeloma is malignancy of plasma cells that reside in the bone marrow and normally function as part of our immune defence system. Plasma cells mature from activated B-cell lymphocytes that are stimulated by foreign substances to produce protective antibodies. However, plasma cells can become cancerous and proliferate out of control forming multiple tumours, called plasmacytomas that excessively overproduce a single abnormal antibody or M-protein. This is multiple myeloma (MM). MM initially responds to chemotherapy, but despite recent advances, virtually all remissions are transient, and the majority of patients relapse and die of their disease.
Many human cancers and notably MM are associated with activation of an oncogene known as c-Myc (MYC). The cMYC protein combines with another MAX to form a complex that binds a DNA regulatory region and promotes the activation of many genes involved in MM cell proliferation. The proliferating cancer cells become addicted and require constant MYC-MAX DNA stimulation to survive. Disrupting this pathway by interfering with MYC-MAX complex formation or MYC-MAX binding to DNA can lead to MM cell death. Recent understanding concerning the biochemical details of MYC-MAX formation led to the discovery of small-molecule inhibiting drugs, such as the compounds reported by Dr. Ed Prochownik from Children’s Hospital in Pittsburgh. These anti-MYC drugs and others like them worked well in bench assays, but unfortunately failed in animal models because the drugs were largely destroyed during circulation in the blood and too little compound reached and penetrated into the MM cells to be effective.
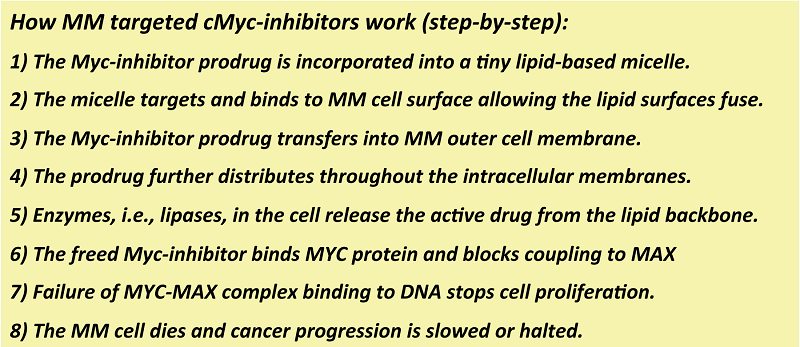
Drs. Gregory Lanza and Michael Tomasson, members of the Siteman Cancer Center at the Washington University School of Medicine in Saint Louis, understood this dilemma and envisioned a novel nanotechnology solution. Their vision was to transform the small molecule cMyc-inhibitors into lipid prodrugs and incorporate them securely into minute micelles that would be home to a common MM cell surface marker, VLA-4. (Figure 1)
Figure 1. How VLA-4-cMyc-prodrug micelles treat multiple myeloma.
The cMyc-inhibitor prodrug was created by using a natural cell membrane constituent, lecithin, and coupling the drug to a modified end of the middle fatty acid (Sn2), the oily part of lipid. Incubation of the cMyc-prodrug MM cell revealed more rapid adsorption of the drug into the MM cell than the free drug. But, unlike the free drug, the cMyc-prodrug is not active as a lipid conjugate. However, once the prodrug enters the cell membranes, the active drug can be liberated into the cytosol by a myriad of enzymes where it can bind the transcription factor, cMyc. This inhibitor, once bound to cMyc, disrupts or distorts its complexation with MAX in the nucleus, and the binding of the oncogenic pair to DNA is disrupted. In this way, the signal for more MM proliferation is blocked and the MM cells, addicted to the cMyc-Max stimulation, die.
Another key advancement was the tiny nanoparticles called micelles, which are not much bigger than an antibody. This small size allows the micelles to penetrate into bone marrow, spleen, and other extravascular MM metastatic depots. To give the micelles MM-specificity, the particles were activated to recognise and bind to the MM cell surface marker, VLA-4. The VLA-4-cMyc-prodrug micelles bypass normal tissues and cells and bind and fuse to almost irreversibly to the MM cells presenting VLA-4. Unlike many nanoparticles, fusion of the micelle lipid surface with the MM cell membrane surface, and the prodrug transfers like a “kiss of death” into the cell. Subsequently, the prodrug passages through the cell membranes into the MM cell, where the active cMyc-inhibitor is freed to have its anticancer effects. This novel drug delivery mechanism is termed “contact facilitated drug delivery” (CFDD).
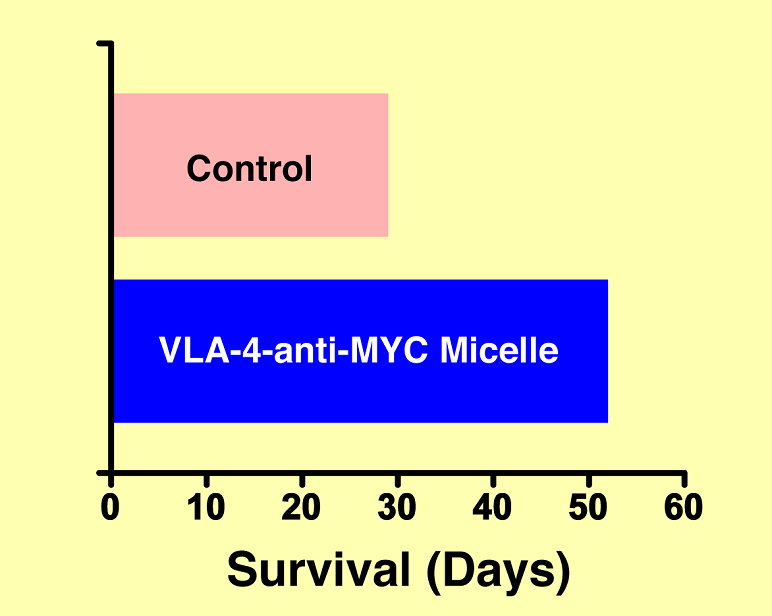
Dr. Tomasson’s laboratory demonstrated the vision of the VLA-4-cMyc-prodrug micelle is an aggressive disseminated mouse model of MM, known as 5TGM1/ KaLwRij. The overarching result of this experiment is shown in Figure 2. The animals that received the VLA-4-cMyc-prodrug micelle system survived nearly twice as long as those mice receiving one of many control or non-targeted treatments. The results were definitive.
Figure 2. Improved survival of mice with multiple myeloma mice given the anti-MYC nanotherapy versus control
Today, Drs. Lanza and Tomasson optimising the nanotherapy using newer cMyc-inhibitors designed and synthesized by Drs. Prochownik, and Steven Fletcher, University of Maryland School of Pharmacy as of the US NCI Cancer Centers of Nanotechnology Excellence (CCNE) program at Washington University School of Medicine, the Siteman Center for Multiple Myeloma Nanomedicine (CMMN).
Gregory M. Lanza
Michael H Tomasson
Department of Medicine, Washington University School of Medicine
Siteman Center for Multiple Myeloma Nanomedicine (CMMN)
greg.lanza@mac.com
Please note: this is a commercial profile