UCL-led trial shows adding niraparib to standard therapy reduces tumour growth risk in men with advanced prostate cancer by up to 37%, with greater effect in specific genetic subgroups
A major international trial led by University College London has unveiled a promising new treatment option for men with advanced prostate cancer. The results suggest that a novel drug combination could help slow disease progression and improve patient outcomes, offering fresh hope for those facing one of the most challenging forms of the condition.
The complete study is published in Nature Medicine.
Overview of the AMPLITUDE Trial
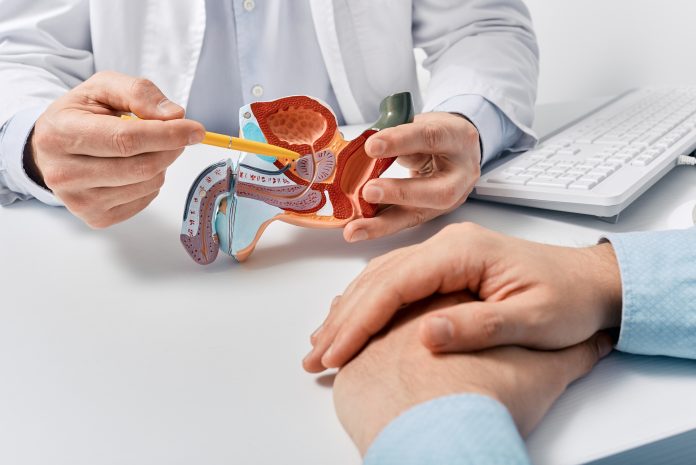
The Phase III AMPLITUDE trial tested the addition of niraparib, a type of targeted cancer drug known as a PARP inhibitor, to the standard treatment of abiraterone acetate and prednisone (AAP). PARP inhibitors work by preventing cancer cells from repairing their damaged DNA, thereby increasing the effectiveness of other cancer treatments. The trial enrolled 696 men across 32 countries with a median age of 68. Half of the participants received the new combination of niraparib plus APP, and the other half received standard treatment with a placebo.
The study focused on patients diagnosed with advanced prostate cancer, where the cells have spread to other body parts, who were starting their first treatment and who also had alterations in genes involved in an essential type of DNA repair, known as homologous recombination repair (HRR).
These genes work by repairing damaged DNA, and when they are faulty, cancer cells can grow and spread more aggressively. Around one in four people with advanced prostate cancer at this stage have altered HRR genes, such as BRCA1, BRCA2, CHEK2, and PALB2. In this study, 55.6% participants had alterations in the BRCA1 or BRCA2 genes.
Niraparib reduced cancer growth by 37%
At a median follow-up of 30.8 months, the researchers found that:
- Niraparib reduced the risk of cancer growth by 37% compared to AAP alone in all patients and by 48% in the subgroup of patients with BRCA1 or BRCA2 mutations.
- The length of time between symptom worsening was twice as long for patients who received niraparib compared to those who received a placebo, resulting in a reduction in the number of patients with notable symptom worsening from 34% to 16%.
- Researchers observed a trend toward improved overall survival in the niraparib group. However, a longer follow-up is needed to confirm that starting niraparib for this patient population enhances life expectancy.
Currently, the standard treatments for advanced prostate cancer, such as abiraterone acetate and prednisone (AAP), are very effective for the majority of patients. However, a small but very significant proportion of patients have limited benefit from these treatments. This is where the new drug combination comes in. By combining with niraparib, we can delay the cancer’s return and hopefully significantly prolong life expectancy for these patients.
“These findings are striking because they support widespread genomic testing at diagnosis with the use of a targeted treatment for patients who stand to derive the most significant benefit.
“For cancers with a mutation in one of the eligible HRR genes, where niraparib has been approved, a doctor should consider a discussion that balances the risks of side effects against the clear benefit to delaying disease growth and worsening symptoms.”