Jan Conn, Sara Bickersmith and Catharine Prussing from the Wadsworth Center, New York State Department of Health and Marta Moreno from the Department of Medicine, University of California-San Diego highlight issues confronting malaria elimination unique to the Amazon Basin…
Broadly defined as all forms of transmission that can persist after achieving full universal coverage with effective LLINs (long-lasting insecticidal nets) and/or IRS (indoor residual spraying), containing active ingredients to which local vector populations are fully susceptible,1 residual malaria requires several caveats in relation to nearly all South American malaria endemic regions. First, both interventions (LLINs, IRS) mainly affect endophagic (indoor feeding) and endophilic (indoor resting) mosquitoes, and the proportion of a mosquito population/species that feeds at night versus during crepuscular hours.2 In the Amazon, as well as other endemic regions globally, more malaria vectors feed and rest outdoors (exophagy and exophily, respectively) than indoors, and thus escape being targeted by either intervention. Second, behavioural heterogeneity is common in many anopheline species.3 Third, full universal coverage is uncommon in South America, although an overall decrease in malaria incidence between 2000-2014 is well documented in every country except Venezuela and, since 2012, in Peru.4 This is not to disparage the use of LLINs or IRS in Latin America, where both have helped to achieve reductions in human-vector interactions, but rather to focus attention on additional local interventions that can be considered in the current climate of malaria elimination efforts.
The primary malaria vector
In the Loreto Department of Amazonian Peru, in the peri-Iquitos region, Anopheles darlingi is the main malaria vector, and here its behaviour is variable: it is mainly anthropophilic, but it also displays opportunism, depending on host availability; it is both exophagic and endophagic; and nearly exclusively exophilic.5 In this region, malaria cases normally peak during the rainy season, January to June (with some variation depending on river basin), corresponding with increased river levels and anopheline density. We have demonstrated also that transmission by An. darlingi is variable at small spatial scales.

Not all village houses are equal
We investigated spatial and space-time patterns of malaria diagnoses for an entire year in one riverine village, Lupuna, consisting of 432 residents in 94 houses along the Nanay River south of the city of Iquitos. In 2014, 177 cases of Plasmodium vivax and 11 of P. falciparum were registered at the Lupuna health center. We found that overall, malaria risk was higher in houses closer to the river.6 The variability detected in malaria risk within a small village during one malaria season is congruent with findings from another recent study in this region,7 and with those across the endemic malaria regions globally. Elucidating this variability, and correlating it with other data, such as mosquito infection rates and breeding site locations, suggests a focus of local malaria control efforts on hotspots within villages could substantially reduce malaria transmission.

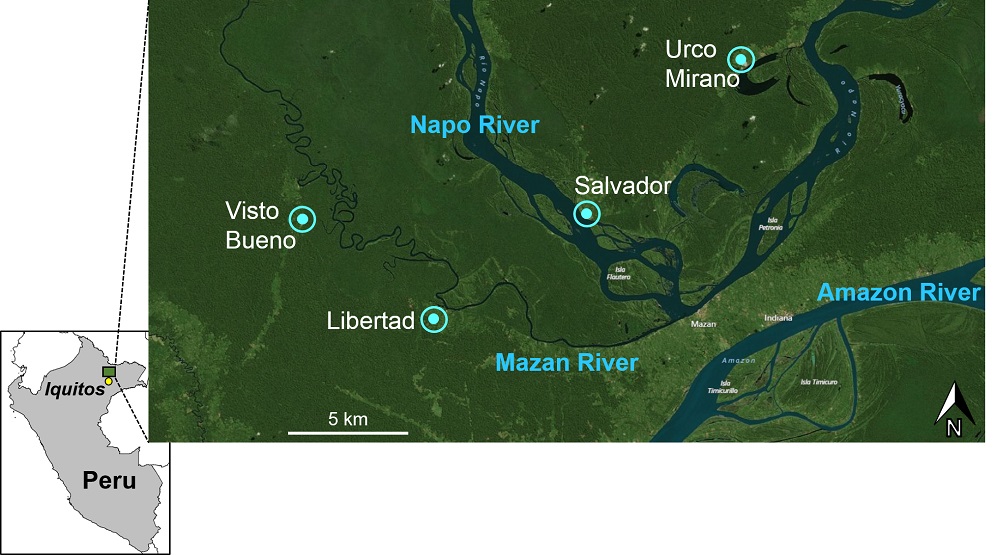
Four study sites along the Mazan and Napo Rivers in Peru
We are focusing on 4 villages, 2 on each of the Mazan and Napo Rivers (Fig. 1). These villages (2015 census, from 65-401 inhabitants) have the highest annual incidence of reported malaria cases for 2014-2015 in the Mazan District, and they are part of a transmission landscape (Figs. 2-3) that is interspersed with remote riverine malaria foci.8 Our research plan, incorporating epidemiology, vector biology and parasitology, may provide data in support of additional interventions such as larval reduction (see below).
Why consider larval reduction?
Many researchers, including ourselves, have found that transmission heterogeneity is widespread at many levels and its causes are complex. They include proximity to breeding site, quality of breeding site, local mosquito species diversity, house construction and host availability. By characterising breeding sites and identifying anopheline species in nearby aquatic habitats (Fig. 4), we are investigating the relationships among several of these factors to evaluate the use of local targeted larval reduction.
References
1 Killeen GF. (2014) Malar J 13:330.
2 Durnez L. (2013) InTech, Ch. 21, pp. 671-704.
3 Smith DL & Coosmans M. (2014) Trans R Soc Trop Med Hyg 108:185–197.
4 WHO World Malaria Report 2015.
5 Moreno M et al. (2015) Malar J 14:290.
6 Prussing C et al. (2015) Abstract LB-5082. Amer Soc Trop Med Hyg Conference.
7 Bautista et al. (2006) Am J Trop Med Hyg 75:1216-1222.
8 Parker BS et al. (2013) Malar J 12:178.
Dr Jan Conn
Vector Biology and Population Genetics Laboratory
jan.conn@health.ny.gov
http://www.wadsworth.org/senior-staff/jan-conn
Please note: this is a commercial profile