Cancer Research UK is backing a £2.06 million, first-in-human trial of LungVax, a preventative vaccine designed to prime the immune system against early lung cancer, a potential breakthrough in reducing the UK’s leading cause of cancer death
In a groundbreaking development, Cancer Research UK is supporting the world’s first clinical trial of LungVax, a vaccine intended to prevent lung cancer rather than treat it. Developed by researchers at the University of Oxford and University College London, the vaccine uses technology similar to that of the Oxford/AstraZeneca COVID-19 vaccine to train the immune system to recognise mutated “red-flag” proteins (neoantigens) on lung cells before they become cancerous.
LungVax: The world-first vaccine aiming to prevent lung cancer before it starts
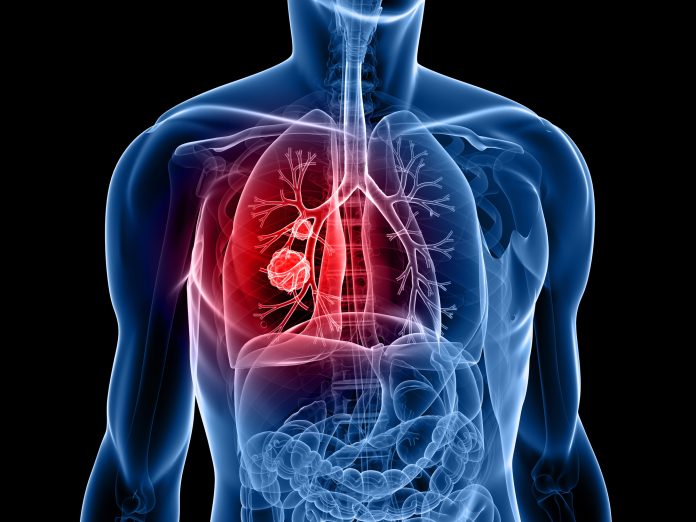
Lung cancer is the leading cause of cancer death in the UK, accounting for 20% of cancer deaths each year. It is difficult to detect early and treat, and only one in ten is diagnosed with the disease.
Researchers from the University of Oxford and University College London have developed a preventive vaccine called LungVax, which has the potential to reduce people’s risk of lung cancer. Lab tests have shown it works by priming the immune system to recognise and kill abnormal lung cells before they become cancerous.
Sarah Blagden, Professor of Experimental Oncology at the University of Oxford and co-founder of the LungVax project, said, “Lung cancer is lethal and blights far too many lives. Survival has been stubbornly poor for decades. LungVax is our chance to do something to prevent this disease actively.”
We’ve awarded the LungVax team up to £2.06 million, supported by the CRIS Cancer Foundation, to initiate testing of the vaccine in a four-year Phase 1 trial, which is expected to commence in summer 2026.
“Years of research into the biology of cancer, understanding the fundamental changes which occur in the very earliest stages of the disease, will now be put to the test,” said Blagden. “This funding means that, for the first time, we hope that people will be able to receive LungVax in clinical trials from next year.”
How the LungVax works
LungVax employs a technology similar to that of the Oxford/AstraZeneca COVID-19 vaccine, which works by providing the immune system with a set of genetic instructions that help it identify and eliminate abnormal cells.
The LungVax identifies cells that are changing in ways that could lead to lung cancer, called neoantigens. Neoantigens are proteins found on the surface of the cell due to cancer-causing mutations within the cell’s DNA.
The purpose of the LungVax is to get the immune system to recognise these abnormal cells early and destroy them before they develop into cancer. This works differently from existing lung cancer vaccines; it prevents the cancer from developing.
Phase 1: Bringing the vaccine to people who need it most
Phase 1 of the trial will determine the optimal dose of LungVax to give people at high risk of lung cancer and examine any possible side effects. It will recruit a small group of people who have already been treated for early-stage lung cancer but have a high risk of their disease returning, along with individuals undergoing targeted lung health checks as part of England’s targeted lung cancer screening programme.
Professor Mariam Jamal-Hanjani of University College London, who will lead the trial, said, “Fewer than 10% of people with lung cancer survive their disease for 10 years or more. That must change, and that change will come from targeting lung cancer at the earliest stages.
“The LungVax clinical trial is the crucial first step in bringing this vaccine to people at the highest risk of the disease. We will be looking carefully at how people respond to the vaccine, how easy it is to deliver, and who might benefit from it most in the future.”