Sonoenhancement with Acoustic Cluster Therapy (ACT®) improves drug delivery to solid tumors, and by that reduces the necessary doses and side effects
Cancer is a devastating disease with high incidence and insufficient treatment. Cancer drugs suffer both from limited effect and acquired resistance by the cancer cells as well as side effects that severely reduce quality of life.
Sonoenhancement with Acoustic Cluster Therapy: The Concept
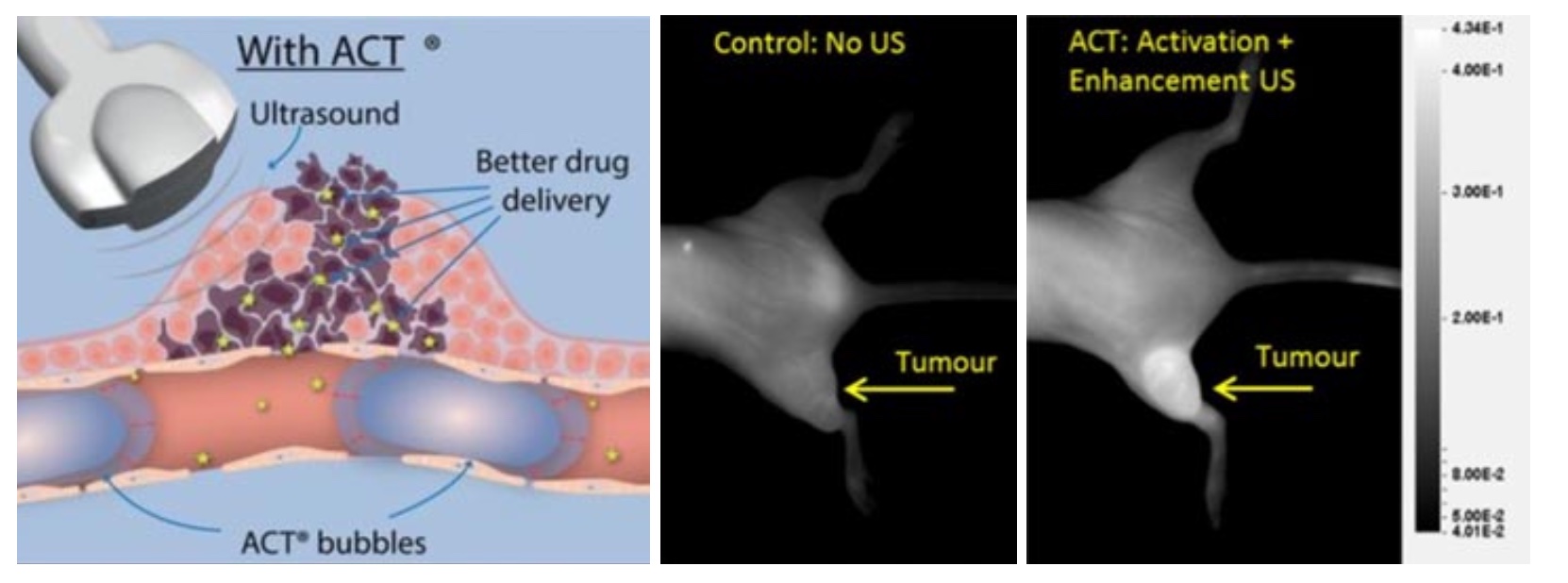
Acoustic Cluster Therapy (ACT®) consists of clusters of gas-filled microbubbles and oil microdroplets. These are administered systemically. Then, a two-step ultrasound sequence is directed towards the tumor giving a controllable and localized therapy. Firstly, the activating insonation at diagnostic imaging frequencies (2 to 10 MHz) causes the gas bubbles to oscillate and the particles in the cluster fuse together. As a result, the oil in the microdroplets is exposed to the gas in the bubbles and evaporates. This results in a large bubbles (20-30 um in diameter) that lodges in capillaries of the tumor. Secondly, the enhancement insonation at 0.5 MHz, the resonance frequency of the large bubbles, makes the larger bubbles oscillate inside the capillaries and transfer energy onto both the blood vessel wall and the surrounding tumor and extracellular matrix and reduce the local barriers to drug delivery (Figure 1). By this minimally invasive process, the tumor is prepped for drug delivery and the effect of the administered drug enhanced.
Pre-Clinical Evidence for Acoustic Cluster Therapy
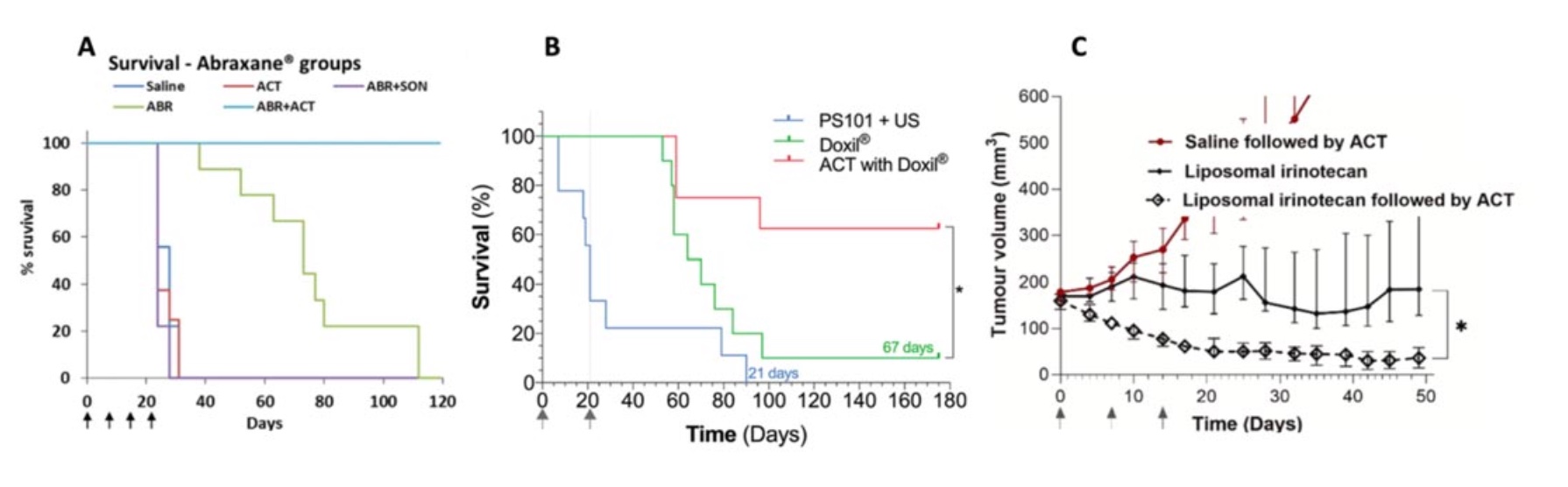
During the last decade ACT® has been evaluated preclinically in various cancer models and combined with different drugs. It was first observed that ACT was able to increase the fluorescence from a tumor when sonoenhancement was combined with fluorescent macromolecules (Wamel 2016, Figure 1). Here it was found that already one minute after sonoenhancement, fluorescence had increased in the tumor compared to a non-treated control, followed by a fluorescence uptake that remained for several hours. Subsequently, ACT has been tested therapeutically in preclinical models of prostate cancer (Wamel 2016), pancreatic cancer (Kotopoulis 2016, Ng 2022), colon cancer (Bush 2019) and breast cancer (Bush 2020). The combination of ACT and drug was significantly better than the drug alone in all these studies, with quite large numbers of complete remissions. Combining ACT with the drug nab-paclitaxel for treatment of prostate cancer resulted in complete tumor remission in all tested animals. This shows that ACT provides sonoenhancement across very different cancer types and with different types of drugs, which increases the likelihood of seeing effects also in clinical trials as the tumor models collectively represents a variety of cancer biology.
Side effects and toxicity have also been tested in various small animal models during the last decade. During treatment, no bleeding or macroscopic damage was observed, and pathological evaluation has not identified microscopic damages. ACT was extensively tested for systemic toxicities, including studies in rats and dogs where ultrasound to the heart and liver was used to activate the ACT bubbles, and no significant adverse effects have been detected.
Sonoenhancement with Acoustic Cluster Therapy
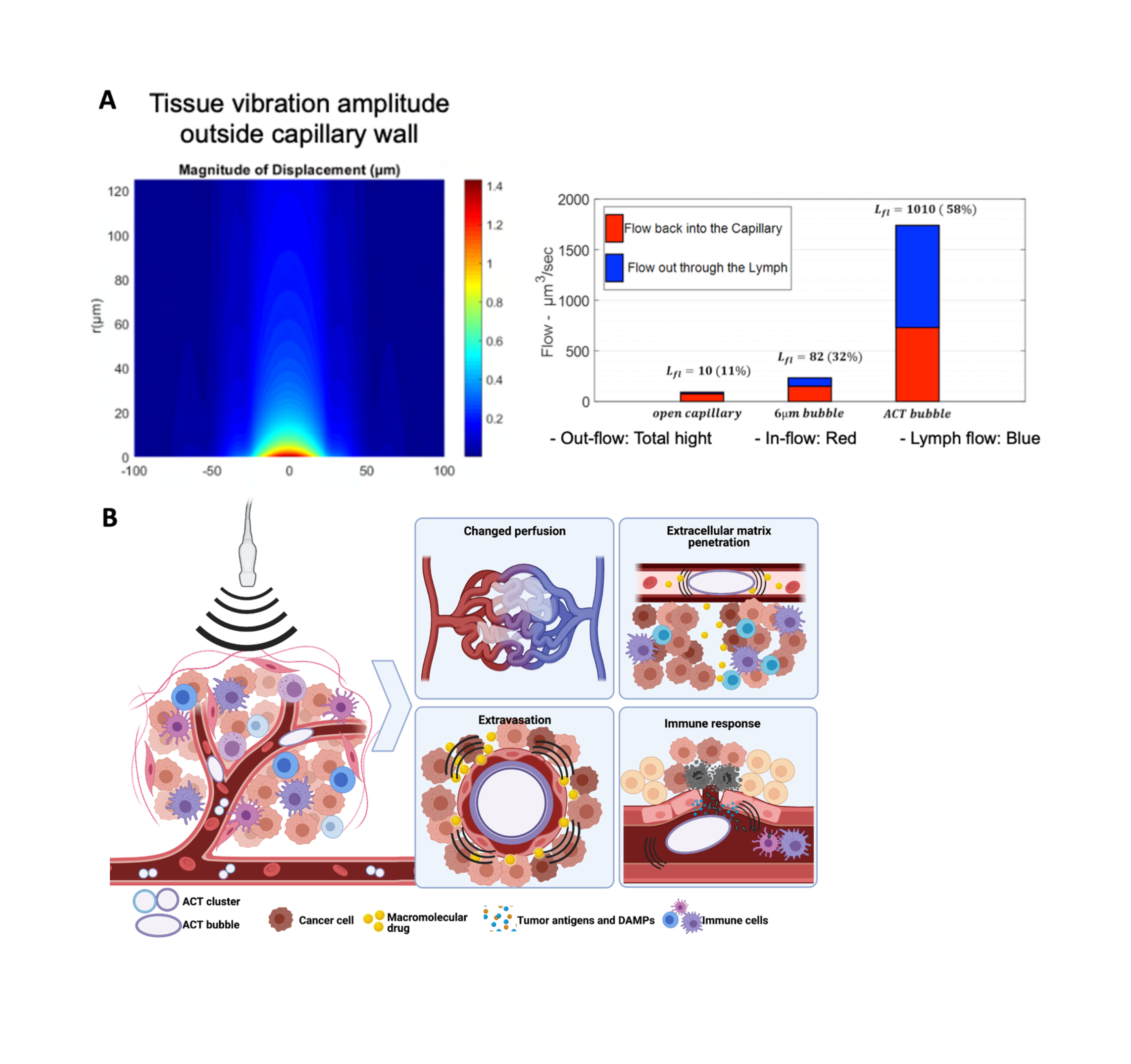
Sonoenhancement describes the actions of ACT. This is distinct from sonoporation describing the mode of action of ultrasound with the conventional free-flowing microbubbles, which are designed for ultrasound contrast enhancement. The mode of action of ACT is that the microclusters expand and lodge in tumor capillaries. This gives multiple effects in the tumor that can be separated into primary and secondary effects. The primary effect of ACT is the oscillations inside the capillaries, which affects the vascular wall and propagate into the extravascular domain of the tumor. This is clearly different from the action achieved with conventional microbubbles. An activated ACT bubble has a volume 1000 times that of a conventional microbubble and a large contact area with endothelial cells. An activated ACT bubble will temporarily block the capillary and oscillations will affect the entire inner surface of the vessel. The volume of the bubble and the amplitude of the oscillations results in biomechanical work that is 1000 times greater than that of conventional microbubbles.
Theoretical modelling suggests that the biomechanical work of an ACT bubble creates both a radiation force and shear waves in the extracellular matrix of the tumor. It is hypothesized that the radiation force can drive fluid streaming, either in a circular pattern back to the vasculature or from blood vessel and into lymphatics (Figure 3). Increased fluid streaming in the tumor may be important for drug delivery, as the pressure gradient and driving force for transport from the vasculature to the tumor is normally scarce. Models have shown that the velocity of fluid streaming can increase from 50 to 1000 nm/s by using ACT.
Shear forces are created by oscillations of inhomogeneities in the tumor and could be especially important in highly stromal tumors such as pancreatic cancer. These waves could break down extracellular macromolecules such as hyaluronic acid which is a barrier for diffusion and transport in the tumor.
Secondary effects of ACT treatment, such as biological responses mounted by the tumor cells and immune system as a response to sonoenhancement, may potentially be the most important for therapeutic benefit. Tumor vasculature is known to be fenestrated due to incomplete coverage by endothelial cells. Increasing these fenestrations likely requires far lower forces than what is required to make fenestrations in healthy vasculature. ACT may thereby increase drug extravasation in a tumor-specific manner. After extravasation, cancer drugs face dense extracellular matrix hindering diffusion. One suggested mechanism of action for ACT is that the shear forces created by ACT can disrupt this matrix and allow for microstreaming that can create semi-stable channels in the tumor that allows for more efficient diffusion and drug transport also after ACT treatment is finalized. In pre-clinical experiments it has repeatedly been observed that drugs can be administered after finalization of ACT treatment with close to similar effect as when given simultaneously with ACT, showing that the effect of ACT on the tumor lasts beyond the actual treatment.
In addition to the effects on delivery, ACT may also be able to stimulate the immune system to facilitate therapy. This can occur either through mechanosensing where the oscillations themselves are detected or as a response to minor damages. The latter most likely as detection of damage-associated molecular patterns (DAMPs) presented by damaged endothelial cells that have been in proximity of an ACT bubble. DAMPs can lead inflammatory immune cells to the tumor and potentiate the effect of immunotherapies such has immune checkpoint inhibition and CAR-T cells.
What is the outlook for ACT effects and potential?
In summary, the drug-enhancing effect of ACT has been proven with multiple drugs and in multiple pre-clinical models. While a lot has been uncovered in terms of therapeutic effect, safety and delivery efficiency, knowledge is still lacking about the extent of contribution of different underlying mechanisms of ACT. This is being pursued in collaborations between Exact Therapeutics who are developing ACT and researchers at ICR London, TGen Phoenix and NTNU in Trondheim. Currently, ACT is being evaluated in clinical trials in colorectal cancer patients with liver metastases in a phase 1 study. Hopefully sonoenhancement with Acoustic Cluster Therapy can be made available to patients outside trials and to other cancer types within the next 3-4 year.
References
- Wamel, A.V. et al. Acoustic Cluster Therapy (ACT) – pre-clinical proof of principle for local drug delivery and enhanced uptake. J Control Release 224, 158-164 (2016).
- Van Wamel, A. et al. Acoustic Cluster Therapy (ACT) enhances the therapeutic efficacy of paclitaxel and Abraxane (R) for treatment of human prostate adenocarcinoma in mice. J. Controlled Release 236, 15-21 (2016).
- Kotopoulis, S. et al. Sonoporation with Acoustic Cluster Therapy (ACT(R)) induces transient tumour volume reduction in a subcutaneous xenograft model of pancreatic ductal adenocarcinoma. J Control Release 245, 70-80 (2017).
- Ng, S. et al. Effect of acoustic cluster therapy (ACT®) combined with chemotherapy in a patient-derived xenograft mouse model of pancreatic cancer. J. Controlled Release 352, 1134-1143 (2022).
- Bush, N. et al. Therapeutic dose response of acoustic cluster therapy in combination with irinotecan for the treatment of human colon cancer in mice. Front Pharmacol 10, 1299 (2019).
- Bush, N. et al. Theranostic attributes of acoustic cluster therapy and its use for enhancing the effectiveness of liposomal doxorubicin treatment of human triple negative breast cancer in mice. Front Pharmacol 11, 75 (2020).

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.