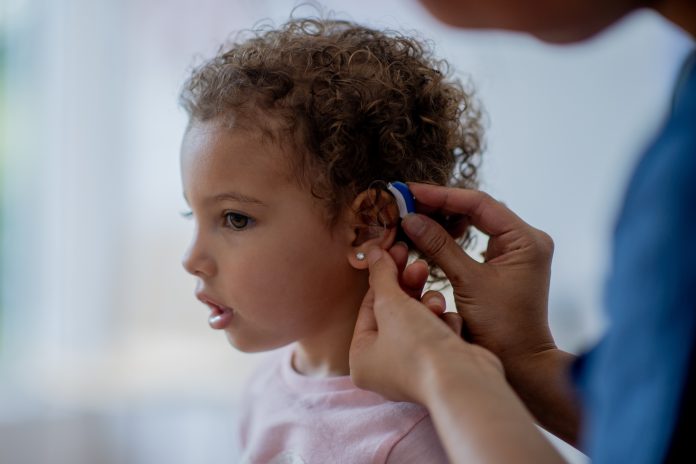
A first-in-patient trial using AAV-based OTOF gene therapy, led by Karolinska Institutet and Chinese hospitals, restored hearing (improving from 106 dB to 52 dB) in 10 individuals aged 1–24, with notable gains in children
A collaborative team from Karolinska Institutet and partner hospitals in China has completed the first clinical trial of a gene therapy designed to treat congenital hearing loss caused by mutations in the OTOF gene. The study, recently published in Nature Medicine, marks a milestone in the development of precision therapies for inherited forms of deafness.
“This is a huge step forward in the genetic treatment of deafness, one that can be life-changing for children and adults,” said Maoli Duan, consultant and docent at the Department of Clinical Science, Intervention and Technology, Karolinska Institutet, Sweden, and one of the study’s corresponding authors.
AAV-delivered OTOF gene therapy restored hearing
The study involved ten patients, aged between one and 24, from five hospitals in China, all of whom had a genetic form of deafness or severe hearing impairment caused by mutations in the OTOF gene. These mutations cause a deficiency of the protein otoferlin, which is crucial for the release of neurotransmitters in the auditory system, playing a critical part in transmitting auditory signals from the ear to the brain.
The gene therapy utilised a synthetic adeno-associated virus (AAV) to deliver a functional version of the OTOF gene to the inner ear. The AAV, which is a harmless virus, was modified to carry the OTOF gene. This modified virus was then delivered to the inner ear via a single injection through a membrane at the base of the cochlea, known as the round window.
The effect of the gene therapy was rapid, and the majority of the patients recovered some hearing after just one month. A six-month follow-up showed considerable hearing improvement in all participants, the average volume of perceptible sound improving from 106 decibels to 52.
Children responded best to the treatment
The younger children, mainly aged between five and eight, responded best to the treatment. One of the participants, a seven-year-old girl, quickly recovered almost all her hearing and was able to hold daily conversations with her mother four months afterwards. However, the therapy also proved effective in adults.
“Smaller studies in China have previously shown positive results in children, but this is the first time that the method has been tested in teenagers and adults, too,” added Dr Duan. “Hearing was greatly improved in many of the participants, which can have a profound effect on their life quality. We will now be following these patients to see how lasting the effect is.”
The findings indicate that the treatment was safe, and the most common adverse reaction was a reduction in the number of neutrophils, a type of white blood cell. No severe adverse reactions were reported in the follow-up period of six to 12 months. Long-term studies are necessary to determine the lasting effects of the treatment, and the researchers will follow these patients to assess the sustainability of the hearing improvement.
“OTOF is just the beginning,” said Dr Duan. “We and other researchers are expanding our work to other, more common genes that cause deafness, such as GJB2 and TMC1. These are more complicated to treat, but animal studies have yielded promising results so far. We are confident that patients with different kinds of genetic deafness will one day be able to receive treatment.”