The UK’s Horizon trial will test a vaccine against the Epstein-Barr virus (EBV), linked to multiple sclerosis, aiming to train the immune system and explore a potential new treatment for MS
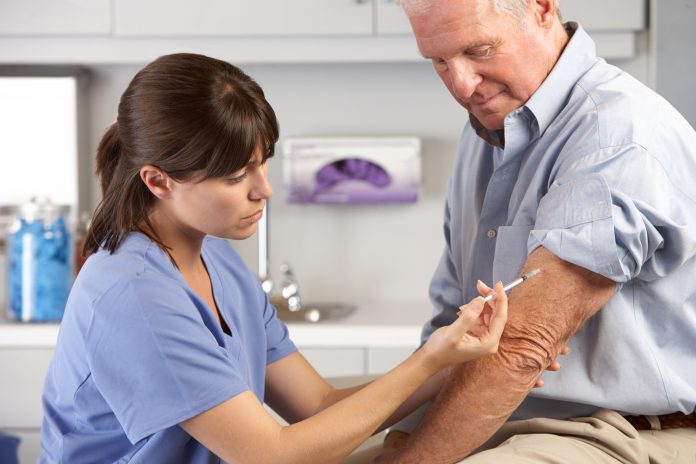
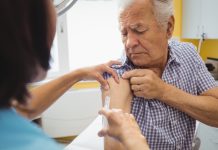
The UK is launching a groundbreaking Horizon trial to see if a vaccine can help people with multiple sclerosis by targeting the Epstein-Barr virus (EBV), a virus found in almost all MS patients. Led by the University of Edinburgh and sponsored by Moderna, the Phase 2 trial will recruit 180 participants across ten UK sites to test the vaccine’s safety and its potential to reduce MS activity. This research could pave the way for a new approach to treating MS by addressing one of its underlying causes.
Horizon trial for MS vaccine
The UK has some of the highest rates of MS in the world. Multiple sclerosis (MS) is a chronic, often disabling disease that affects the brain and spinal cord. It cannot be cured and its symptoms can be debilitating, including fatigue, numbness in the body, memory problems, and muscle cramps.
The Horizon trial will involve an investigational vaccine, and it will be trialled in 180 patients recently diagnosed with MS. The trial will involve up to ten sites across the UK, led by the University of Edinburgh, and is sponsored by the pharmaceutical company Moderna.
EBV usually causes no symptoms but often leads to glandular fever. Once infected, the virus remains in the body for the rest of one’s life but can become active again. The vaccine aims to train the immune system to keep the EBV virus suppressed, which is thought to be a potential underlying cause of MS.
Expert commentary
“This is an important and innovative trial to treat multiple sclerosis by targeting EBV infection using a vaccine. Currently, almost all of our disease-modifying treatments for multiple sclerosis work by suppressing the body’s immune system. The discovery that EBV plays an important role in the development of multiple sclerosis is opening new avenues for treating the condition,” commented Professor David Hunt, National Chief Investigator for the trial and Director of the MS and Neuroimmunology Hub at the Anne Rowling Clinic.
“The partnership between the University of Edinburgh, the National Institute for Health and Care Research and NHS Research Scotland has enabled the UK to be the first country outside the US to open the study, affording more UK patients the choice to participate in the trial. That is a vital step which could potentially lead to a significant breakthrough underpinned by Scottish leadership,” added Professor Dame Anne Dominiczak, Chief Scientist (Health), Scottish Government.
“This new Horizon Trial, targeting the Epstein-Barr virus, exemplifies the groundbreaking work being undertaken as part of the Government’s strategic partnership with Moderna, led by the UK Health Security Agency. Not only does this Partnership provide additional resilience to respond more effectively to any future pandemic, but it also opens doors to working closely with academia to develop new vaccine products for a range of existing health conditions. If successful, these would help support the Government in its delivery of the 10 Year Health Plan and the focus on prevention,” commented Sarah Collins, Director of Commercial, Vaccines & Countermeasures Delivery at UK Health Security Agency.