Dr. Carlos Ziebert, head of IAM-AWP’s Calorimeter Center, KIT, explains a calorimetric method for internal pressure measurement, which helps paving the way for safer Lithium-ion cells
Established in 2011 the Calorimeter Center at the Karlsruhe Institute of Technology’s (KIT) Institute for Applied Materials – Applied Materials Physics, operates Europe’s largest battery calorimeter laboratory. With six Accelerating Rate Calorimeters (ARCs, Thermal Hazard Technology) of different sizes used in combination with cyclers, our team can quantitatively evaluate the thermodynamics, thermal and safety data for Lithium-ion cells on the material, cell and pack levels under quasiadiabatic and isoperibolic environments for both normal and abuse conditions (thermal, electrical and mechanical). Lithium-ion cells have the advantages of high energy density, high open circuit voltage, fast charge / discharge, no memory effect and low self-discharge, which make them the most suitable power source for portable electronic devices and for the electrification of transport, which is more and more impacting our daily lives in the 21st Century. Recently we have developed a calorimetric method to measure the internal pressure of these cells.
Safety first
Safety comes first – this is the mission of the Center’s head, Dr. Carlos Ziebert. A holistic safety assessment is a prerequisite for upscaling and market acceptance of battery technologies, because an uncontrollable increase in temperature of the entire system (so-called thermal runaway) can cause ignition or even explosion of the cell that leads to negative public attention or even rejection. With increasing energy density the safety of Li-ion batteries is becoming more and more important. Thus calorimetry is a fundamental technique in order to obtain quantitative data on the thermal and safety behaviour – you need to know how many Watts a cell will produce under every condition. This information can be used to adapt battery management, thermal management and safety systems.
Method for internal pressure measurement
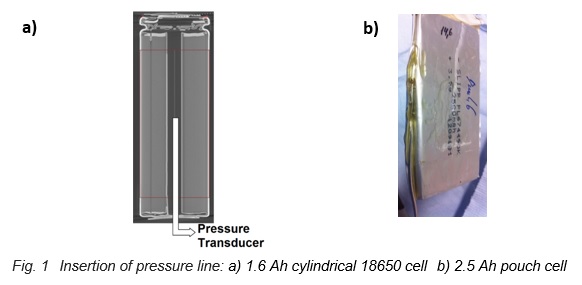
The internal pressure is an important parameter, because its increase can be used as an early warning signal for thermal events that later might lead to the thermal runaway. A new calorimetric method for the measurement of internal cell pressures has been established on 18650 cells and has recently been transferred to pouch cells. A pressure line connected to a pressure transducer is directly inserted into the cell, as shown in the X-ray tomography image of an 18650 cell in Figure 1a) and on the left side of the pouch cell in Figure 1b). Then the cell is sealed again with epoxy resin and the cell is placed into the calorimeter chamber, which has heaters and thermocouples located in lid, bottom and side wall. Finally, the internal pressure is measured during the so-called Heat-Wait-Seek (HWS) test [1]. This test starts in the Heat mode by heating up the cell in 5 K temperature steps. At the end of each step the Wait Mode is activated to reach thermal equilibrium. Then the system enters Seek Mode, which seeks the temperature rate and ends with two possible modes – Exotherm Mode or Heat Mode. If the measured temperature rate is larger than the onset sensitivity (typically 0.02 K/min), the system goes into Exotherm Mode. This mode represents quasiadiabatic conditions, which means that the temperature of the calorimeter follows instantaneously the surface temperature of the cell; that is, the cell cannot exchange heat with the surroundings anymore. Thus, it is heating up more and more until the thermal runaway occurs or the chemicals are completely consumed by the exothermic reactions. On the other hand, if the temperature rate is smaller, the system goes back into Heat Mode.
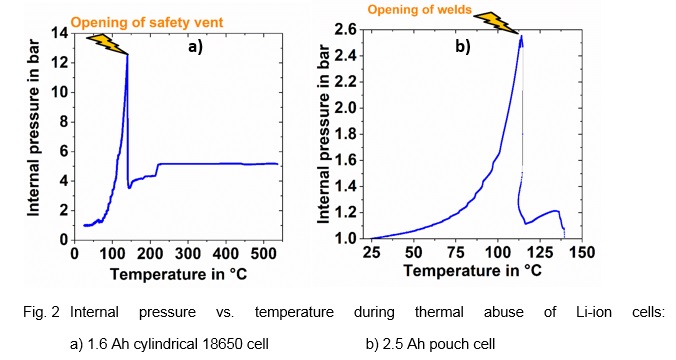
The resulting plots clearly show the internal pressure increase. In the case of the 18650 cell this leads to the opening of the safety vent (s. Fig. 2a)), when a critical pressure of 12.5 bar is reached [2]. For the pouch cell the welds open at a pressure of around 2.6 bar and a temperature of around 110 °C. This method can also be adapted to large prismatic automotive cells. The next step would be to develop a pressure sensor that could be integrated into the Battery Management System (BMS) to act as an additional safety feature.
References
[1] C. Ziebert, A. Melcher, B. Lei, W.J. Zhao, M. Rohde, H.J. Seifert, Electrochemical-thermal characterization and thermal modeling for batteries, in: L.M. Rodriguez, N. Omar, eds., EMERGING NANOTECHNOLOGIES IN RECHARGABLE ENERGY STORAGE SYSTEMS, Elsevier Inc., ISBN 978032342977 (2017).
[2] B. Lei, W. Zhao, C. Ziebert, A. Melcher, M. Rohde, H.J. Seifert, Experimental analysis of thermal runaway in 18650 cylindrical cells using an accelerating rate calorimeter, Batteries 3 (2017) 14, doi:10.3390/batteries3020014.
Dr. Carlos Ziebert
Head of the Calorimeter Center
Karlsruhe Institute of Technology
Institute of Applied Materials – Applied Materials Physics

Tel: +49-721/608-22919







