Senior Research Analyst for Frost & Sullivan Divyaa Ravishankar discusses the growing need for innovative products in the realm of bio-storage applications.
The concept of biobanking has triggered massive interest in the area of long-term sample storage conditions but with a key challenge of maintaining sample integrity. In order to combat this, biobanks are adopting new storage methodologies and solutions that will guarantee better sample quality to the research community.
Globally, sample storage is an outsourced activity by many large pharmaceutical companies. Commercialisation of biobanking activity has forced the providers to adopt tools that are more sophisticated and facilitate sample tracking. Laboratory information management systems (LIMS) prove to be an essential component in facilitating various biorepository models and it is important to understand the workflow involved in each biobank set up; this will aid the adoption of automation at certain levels.
Market Insights
Researchers handling small quantities of samples are at the risk of getting contaminated. Further, maintaining consistency becomes a huge factor when large quantities of samples are processed. Therefore, automated protocols are replacing manual ones.
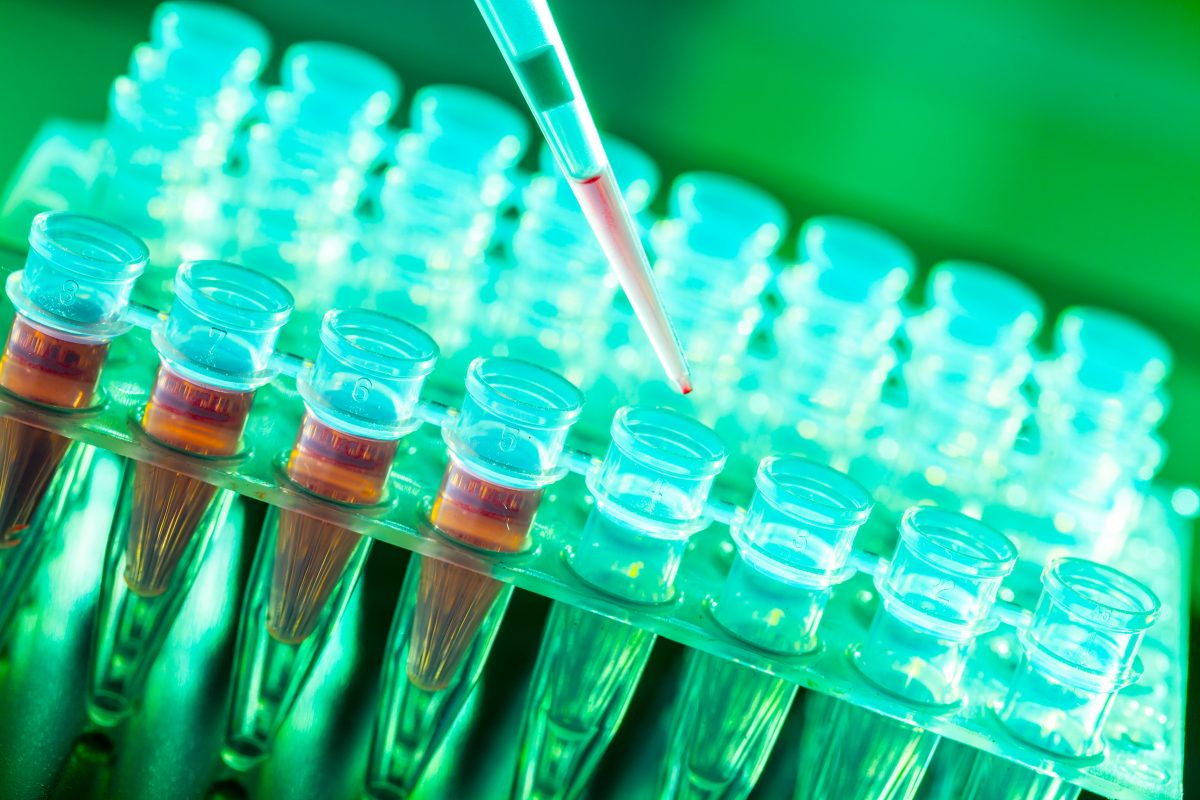
Interest in dry-state storing and eliminating freeze-thaw cycles causing unwanted intervention of sample quality has brought many patented automated biobanking storage platforms for -20°C and -80°C with a facility to store samples in both microplates and micro-tube format. Continuous monitoring of samples is ensured even during picking.
The cornerstone of every biorepository lies in the efficiency of its freezer inventory software or the LIMS employed. The key objective is to enable researchers to locate and use biospecimens. Besides tracking the location of the vial of a specific sample, it is important to retrieve the associated additional data such as consent information, demographic information and related regulatory data.
Challenges Associated with Clinical Sample Storage
Primitive methods of storing samples in cryotanks have reported instances of loss of samples, with them either being discarded, owing to the fact that they become unidentifiable, or due to ‘handling errors’. The sample retrieval process would be laborious if performed by humans, with the loss of an ID label leading to sample mix-up.
Given the fact that no 2 biobanks function in a similar way, it is tough to generalise a technology platform that is common for them. A lot of custom work is required to suit the workflow processes of a biobank, and at the same time, funding and financial maintenance of the biobanking infrastructure becomes tougher in the long run.
With time, samples demand more sophisticated methods of storage with clinical samples requiring a highly integrated set up that involves continuous monitoring of temperatures, along with the associated sample information.
An exponential increase in the volume of samples is leading to issues with store capacity and duration, with space to accommodate new samples in the given temperature and conditions posing a huge problem.
Today, the lack of high-quality and clinically annotated samples is seen as a major drawback. There is a need for standardising sample handling and storage protocols globally. Owing to very few standardised quality checking protocols for the pre-analytical phase, there arises a difficulty to compare and share samples, especially when specimen volumes are likely to be high. These issues need to be addressed, as they prove to be a barrier for the development of new treatments.
Many issues associated with the scientific use of biobanking samples are ethical in nature, such as consent, personal integrity, privacy protection, safety of samples and access to data and stored samples. The laws and regulations pertaining to ownership, intellectual property rights and commercialisation discourage the use of resource material. There are also issues pertaining to cross-border shipping of samples, which requires consent from donors. With the sole aim of safeguarding the donor information, biobanking acts in Norway and Sweden allow the analysis of samples but discourage their long-term storage.
Technology Innovations for Clinical Sample Storage
Biobanks seek solutions that are easy, efficient and are able to provide cost-effective sample management. Traditional methods of storage include storing samples in laboratory freezers at -20°C, -80°C and liquid nitrogen, and this process is being largely automated with the help of RFID and the MEMS technology.
On the other hand, the recent trend shows an increasing preference towards room temperature storage. There are firms developing reagents to stabilise the DNA and RNA in order to be able to last long under ambient temperature; this concept has allowed whole blood samples to be shipped and preserved under room temperature for about 3 months. Adapting to room temperature storage can yield benefits such as eliminating the need for freezer units and extra storage space.
Over time, it becomes tough for biobanks and biorepositories to track and retrieve samples when stored at ultra-low temperatures. Traditional methods of storage involve microplates with barcode readers. Retrieval of a single sample from a microplate meant thawing the entire plate, which affects the freeze-thaw cycles of other samples simultaneously. For this purpose, sample storage is being carried out in microtubes and individual vials. Earlier, equipment and robotic arms were designed to handle microplates; now, systems are flexible to cherry pick individual microtubes. Most of the storage systems today provide robotic interfaces inside a chilled atmosphere in order to prevent the disturbance of unused samples.
Conclusions
A totally integrated system of hardware, software and consumable tools would be the way to “smart biobanking”. With many new technologies, biobanks oriented towards the future can retain sample quality/integrity by employing smart and smooth sample handling systems available in the market today.
Divyaa Ravishankar
Senior Research Analyst, Life Sciences
Frost & Sullivan