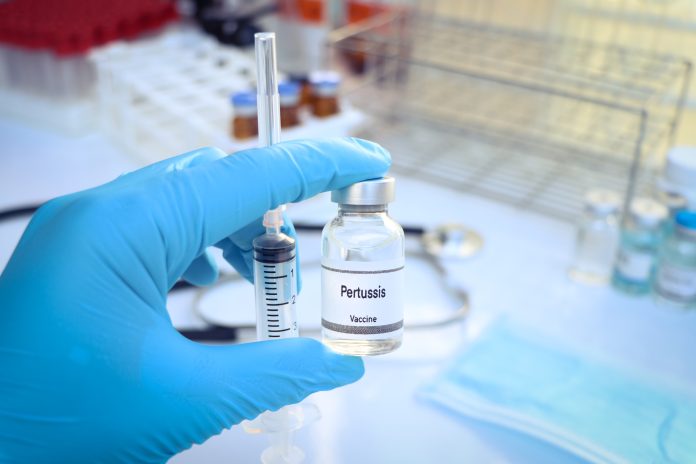
A NIHR-backed trial has found that BPZE1, a nasal spray vaccine, can block whooping cough bacteria from colonising the nose and throat, offering hope for preventing transmission and limiting outbreaks
A new study backed by the NIHR reports encouraging early results for a nasal vaccine designed to better control whooping cough. Researchers say the experimental treatment showed promising signs in a controlled human challenge trial, suggesting it could play an important role in preventing future outbreaks. Further testing will now determine how effective it could be on a wider scale.
The results are now published in The Lancet Microbe.
Rising whooping cough cases highlight the need for new vaccines
In 2024, the UK Health Security Agency (UKHSA) found that whooping cough cases were at their highest level in 30 years. There were 14,879 cases, the highest number in England since enhanced surveillance started in 1994.
The Annual Pertussis Report for 2024: Laboratory-confirmed cases of pertussis in England showed that whooping cough peaks every 3-5 years; however, COVID-19-related social distancing kept disease levels down in 2020-2023.
Despite the availability of vaccines as part of the routine childhood vaccination programme, current options do not provide lifelong protection or effectively stop people from carrying or spreading the bacteria.
This makes new strategies even more crucial. Whooping cough is a serious disease that affects millions of people worldwide. The vaccine, named BPZE1, was tested in a clinical trial at the NIHR Southampton Biomedical Research Centre (BRC) and the NIHR Southampton Clinical Research Facility (CRF). It can be very dangerous for young babies, especially before they get their first vaccine doses at 8, 12, and 16 weeks in the UK programme.
BPZE1 nasal vaccine shows promise in blocking whooping cough bacteria
The researchers found that the vaccine BPZE1 can prevent the bacteria that cause whooping cough from living in the nose and throat, a key step in preventing the spread of infection.
The CHAMPION-1 study tested whether BPZE1, a weakened version of the whooping cough bacterium, could safely protect people from infection and was administered as a single nasal spray.
53 adult volunteers took part in the trial, which exposed them to the whooping cough bacteria 2 to 4 months after receiving the vaccine or placebo. They stayed in a quarantine facility for 16 nights and were given antibiotics to clear the remaining bacteria. Researchers monitored their health and collected samples.
The researchers found that BPZE1 was safe and well-tolerated, with no serious adverse effects. Most people who received the vaccine had little or no bacteria in their noses after being exposed. This means they could be less likely to pass the infection on to others. The vaccine also triggered strong immune responses in both the nose and the blood. This could suggest it offers long-lasting protection.
Public Health Minister Ashley Dalton said: “This government-supported trial marks a major breakthrough in our fight against whooping cough.
“Unlike the vaccine currently offered to pregnant women, which works by protecting babies before birth and is shown to prevent most whooping cough deaths in infants, this new nasal spray vaccine acts differently. It prevents bacteria from living in the nose and throat, which could help reduce transmission and protect more people, not just newborns.
“It’s a powerful showcase of the UK’s world-class research sector, driving innovation to protect future generations.”
Professor Robert Read, who led the study at the NIHR Southampton BRC, said: “This is the first time a whooping cough vaccine has been shown to prevent the bacteria from colonising the nose and throat in humans. That could represent a big step forward in stopping the spread of the disease.”
The trial was designed using a world-first whooping cough-controlled human-infection model developed at the University of Southampton as part of the international PERISCOPE consortium.
Dr Diane Gbesemete, Principal Investigator at the NIHR Southampton BRC, said: “Despite high vaccination rates, outbreaks persist. This study shows BPZE1 could offer better protection and reduce transmission, supporting stronger disease control overall.”
Professor Marian Knight, Scientific Director for NIHR Infrastructure, said: “This important research shows how bringing together industry and our world-leading NIHR infrastructure researchers leads to crucial, globally-significant discoveries. This study takes us a step closer to stopping the spread of whooping cough and eventually eradicating it altogether. This shows how NIHR research, funded by the public, is at the frontier of protecting us from emerging health threats.”
There is no clinical trial data on BPZE1 for mothers yet. However, it plans to conduct additional preclinical studies and later clinical trials to support the use of BPZE1 during pregnancy.