A dangerous hospital superbug, Pseudomonas aeruginosa, can now digest medical plastic like sutures and implants, microbiologists reveal. This alarming ability allows the pathogen to survive longer and form tougher antibiotic-resistant biofilms, posing a significant new threat to patient safety in healthcare settings
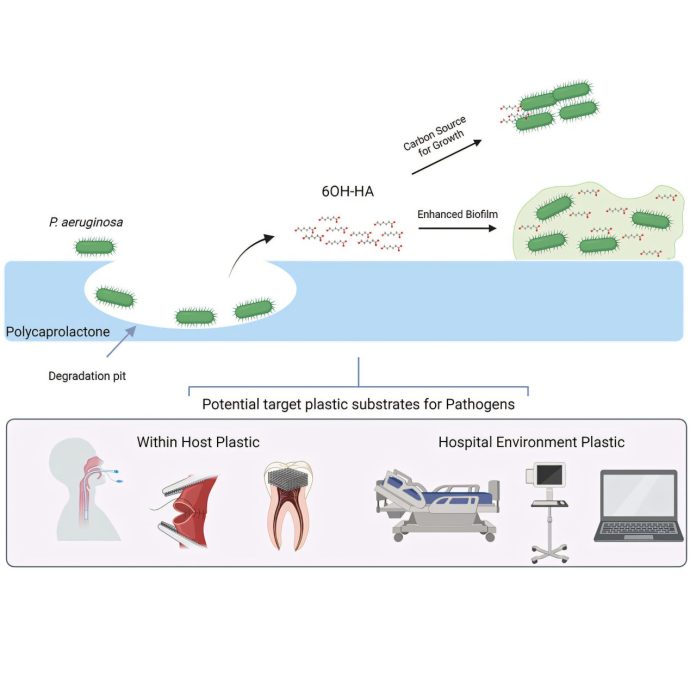
A concerning discovery from microbiologists at Brunel University of London has unveiled a dangerous new capability in a common hospital superbug. Their research, published in Cell Reports, demonstrates that Pseudomonas aeruginosa, a notorious antibiotic-resistant bacterium, can digest polycaprolactone (PCL), a plastic widely used in various medical devices such as sutures, stents, and implants.
This alarming finding challenges the long-held assumption that medical plastics are impervious to microbial degradation and carries significant implications for infection control and patient safety.
The ability of this plastic-digesting superbug to metabolise plastic for survival raises concerns about its persistence in hospital environments and the potential for it to compromise the integrity of medical treatments.
Plastic consumption enhances bacterial resilience
The Brunel University team isolated an enzyme, aptly named Pap1, from a patient-derived strain of Pseudomonas aeruginosa. Laboratory tests revealed the remarkable efficiency of this enzyme, which degraded a substantial 78% of a PCL sample within a mere seven days. Even more concerning was the finding that the bacteria could utilise the plastic as their sole carbon source, essentially consuming it to fuel their growth.
This newfound ability to feed on plastic could significantly extend the lifespan of these pathogens on plastic surfaces within hospital settings, potentially contributing to the spread of infections.
Tougher biofilms and increased virulence
The implications of this plastic-digesting capability extend beyond mere survival. The researchers discovered that the fragments resulting from the breakdown of PCL actually aided the bacteria in forming more robust biofilms.
These biofilms are tenacious, slimy layers that bacteria produce to protect themselves from antibiotics and the host immune system, making infections notoriously difficult to eradicate. By facilitating the formation of these tougher biofilms, the superbug’s plastic-eating prowess directly contributes to its virulence and its ability to resist treatment.
Plastic-digesting superbug: Broad implications for medical materials
While the initial study focused on PCL, the researchers identified indicators of similar enzymes in other pathogenic bacteria. This suggests that the vulnerability to microbial degradation may not be limited to PCL alone. Crucially, this raises concerns about the potential susceptibility of other commonly used medical plastics, including polyethylene terephthalate and polyurethane, which are found in a wide array of critical medical devices such as bone scaffolds, dental implants, catheters, and even breast implants.
This broader potential for degradation necessitates a re-evaluation of the materials used in medical applications and the strategies employed to prevent hospital-acquired infections.
Rethinking infection control strategies
Professor Ronan McCarthy, who led the study, emphasised the urgent need to reconsider how pathogens persist in hospital environments. The possibility of plastic surfaces acting as a food source for these bacteria necessitates a re-evaluation of cleaning and disinfection protocols. Furthermore, McCarthy suggests the potential benefit of focusing on developing medical-grade plastics that are inherently more resistant to microbial digestion and implementing screening for these plastic-degrading enzymes, particularly in cases of unexplained and prolonged hospital outbreaks.
As plastic is ubiquitous in modern medicine, understanding and mitigating the impact of these plastic-degrading pathogens is paramount to safeguarding patient well-being. Further research is now critical to fully comprehend the prevalence of these enzymes and their broader implications for infection control and the longevity of medical devices.