Peter H. Santschi, Regents Professor at the Department of Marine Sciences, Texas A&M University – Galveston discusses radioiodine in the environment, focussing on the importance of natural organic matter
There is a single stable isotope of iodine, 127I, and two environmentally relevant iodine radionuclides, 131I and 129I, both of which have been primarily introduced into the environment because of human activity since the dawn of the nuclear age. 131I has a half-life of just eight days (the duration required for it to radiologically decay to half of its mass) but is a threat to human health immediately following a nuclear accident, such as Chernobyl or Fukushima, because of its high inventory, and bio-concentrates in the thyroid gland. Because of its short half-life, 131I levels usually fall below detection in environments contaminated by nuclear event shortly after the release (about two months).
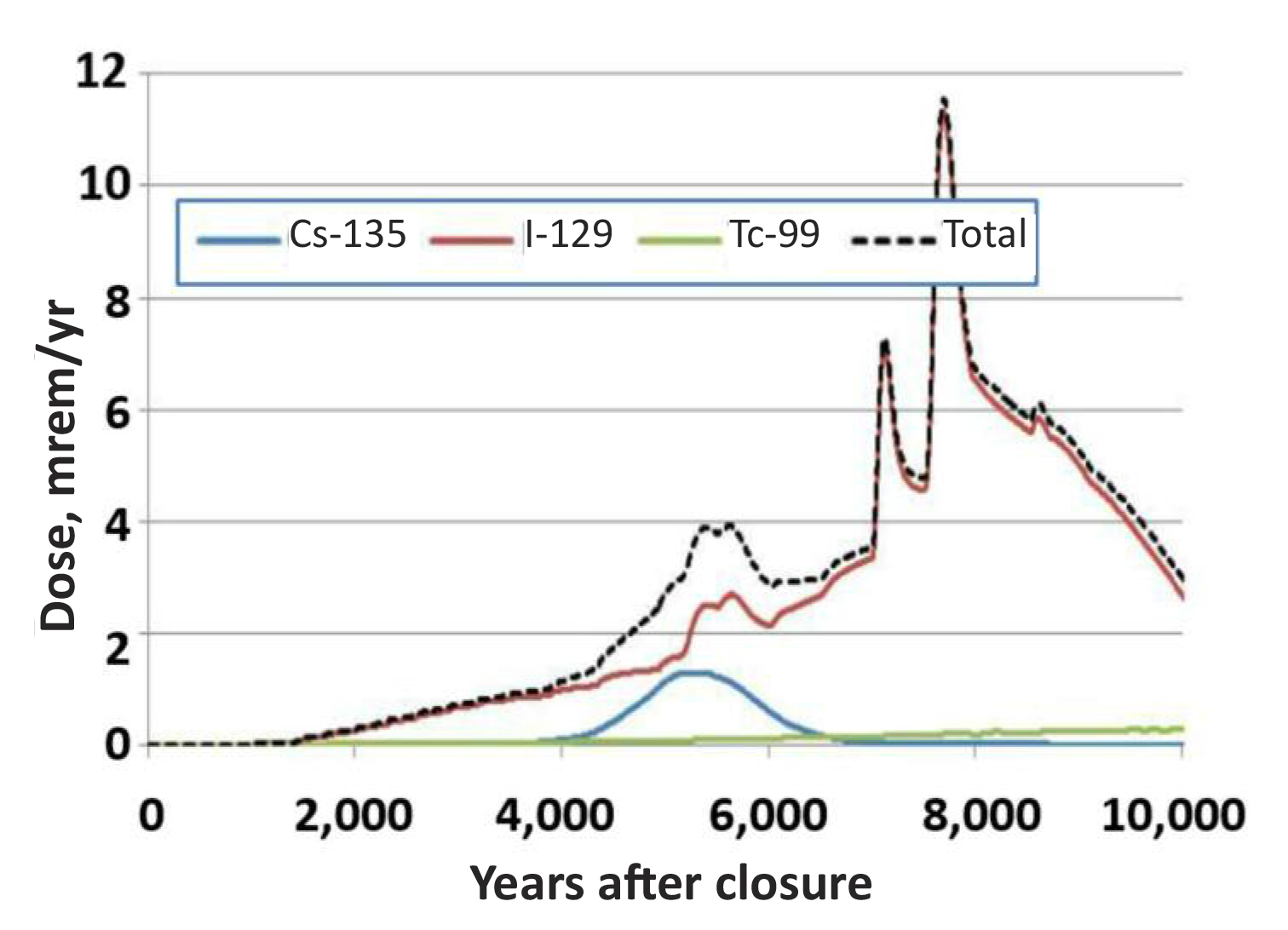
In contrast, 129I is much less of an immediate health risk because it has a much longer half-life (16 million years), however, it is problematic as a contaminant associated with nuclear waste disposal. Another important reason that 129I is a key risk driver is that there is the uncertainty regarding its biogeochemical fate and transport in the environment and such uncertainty requires that conservative assumptions about its associated risk must be included in our risk models. Its risk is considerably higher than that of 99Tc, which receives most of the attention in the US [Fig. 1].
As a consequence of some of these characteristics, 129I has a very low Drinking Water Standard, DWS, which is set at 0.04 Bq/L, the lowest of all radionuclides in the US Federal Register. Thus, 131I is a significant and immediate health hazard associated with large-scale nuclear events, whereas 129I poses a challenge in terms of environmental remediation and the long-term stewardship of nuclear waste (Kaplan et al., 2014).
Stable iodine is a required nutrient for human health. About 90% of the iodine in the human body exists in the 14-g thyroid gland, where it is an essential component of several thyroid hormones. When radioiodine enters the human body, it mimics the behaviour of stable iodine and concentrates on the thyroid gland but can be a carcinogen.
The World Health Organization (2006), reported that the Chernobyl nuclear power plant accident resulted in 5,000 thyroid cancer cases of people who were under 18 years old at the time of the accident. Radioactive iodine was deposited in pastures eaten by cows who then concentrated it in their milk which was subsequently drunk by children. This was further exacerbated by a general iodine deficiency in the local diet causing more of the radioactive iodine to be accumulated in the thyroid. Since radioactive iodine is short-lived, if people had stopped giving locally supplied contaminated milk to children for a few months following the accident, it is likely that most of the increase in radiation-induced thyroid cancer would have been averted.
Iodine exists in multiple oxidation states, primarily as molecular iodine (I2), iodide (I-), iodate (IO3-) or organic iodine (org-I). The mobility of iodine in the environment is dependent upon its speciation and a series of redox, complexation, sorption, precipitation and microbial reactions. Over the last 15 years, there have been significant advances in iodine biogeochemistry and geobiology, largely spurred by a renewed interest in the fate of radioiodine in the environment and advances in detecting these various species at environmentally relevant concentrations. The biogeochemistry of iodine, with particular emphasis on the microbial processes responsible for volatilisation, accumulation, oxidation and reduction of iodine, are reviewed in Yeager et al. (2017).
The key environmental factor influencing radioiodine’s fate and transport in the environment is natural organic matter (NOM) that consists of residues of decaying plant matter and freshly produced exudates from microbes (Santschi et al., 2017a). Recently, great progress has been made in understanding the impact NOM compounds and microbial processes on the fate of different radionuclides in Japanese and U.S. soils. It has been shown that NOM not only influences the fate and transport of radioiodine but also many other radionuclides. However, there still remains great uncertainty in predicting NOM-radionuclide interactions because of a lack of understanding of radionuclide binding to the wide array of specific binding sites (organic moieties) within NOM, as NOM is polymeric, polyfunctional, containing pH and redox reactive moieties that can be controlling radionuclide behaviour in the environment.
The architecture of macromolecular organic matter requires that thermodynamic constants for binding to macromolecular organic matter will have distribution functions rather than discrete values, which is a challenge for modellers. While radionuclide-NOM studies have been conducted using model organic compounds or elevated radionuclide concentrations, the results of such studies might provide compromised information related to true environmental conditions.
Therefore, sensitive techniques are required not only for the detection of radionuclides and their different species, at ambient and/or far-field concentrations, but also for potential trace organic compounds that are chemically binding these radionuclides (Santschi et al, 2017b). Recent analytical chemistry advances (based on GC-MS and AMS) have demonstrated that iodine forms strong bonds with NOM by covalently binding to aromatic functionalities. These recent studies have led to a more mechanistic understanding of radioiodine biogeochemistry.
Different from other high risk radionuclides (Cs, Sr, and U) that have been attenuated, 129I continues to leave the source at a rate that may have been exacerbated by the initial remediation actions (Kaplan et al., 2014) that ignored the strong pH and redox control of its organoiodine formation and mobilisation/immobilisation that is opposite to that of many metal radionuclides.
In another example, the Fukushima Prefecture surficial soil 127I content was significantly and positively correlated to soil OM content, regardless of land use type and showed strong correlations (negative to pH, positive to Eh) to soil 127I content, suggesting that soil OM might be an important factor affecting iodine biogeochemistry. These observations have far-reaching implications for remedial actions and demonstrate the need for additional understanding of the impact of NOM interactions on the fate and transport of radioiodine.
Acknowledgements
Work on radioiodine reported here was funded by the U.S. Department of Energy (DE_SC0014152.), while some work on other radionuclides by the U.S. National Science Foundation (OCE0851191)
References
Kaplan, D. I., et al. 2014. Critical Reviews of Environmental Science and Technology, 44(20), 2287-2335.
Santschi, P.H., et al. 2017a. Appl. Geochem., 85, 121-127.
Santschi, P.H., et al. 2017b. J. Environ. Radioactivity, 171, 226-233.
Yeager, C.M., et al. 2017. Advances in Applied Microbiol., 101, 83-136.
Cancer consequences of the Chernobyl accident: 20 years after
Journal of Radiological Protection (. vol 26(2), pages 125- (on-line doi:10.1088/0952-4746/26/2/001).
Please note: this is a commercial profile
Peter H. Santschi
Regents Professor
Department of Marine Sciences
Texas A&M University – Galveston
Tel: +1 409 740 4476











