Dr Alfred Msezane from the Department of Physics, Clark Atlanta University, provides new insights on physics, in particular, on metastable and excited states negative ion formation in fullerene molecules
Here, we focus our theoretical exploration on the negative ion formation in low-energy electron elastic collisions with fullerene molecules. Fullerenes have extensive and crucial applications in science, nanotechnology and industrial research, as well as in astrophysics. In particular, understanding the stability and degradation mechanism in modern organic solar cells is essential before their commercialisation. Towards this end, designing polymers and fullerenes, the working materials, with larger electron affinities (EAs) have been
proposed. Interest in the fullerene molecules here is dictated by the existence of high quality measured EAs, from C20 through C92.
This has allowed us to investigate ground state electron scattering from the C20 through C240 fullerenes. (1, 2) Where the measured EAs are available, our Regge-pole calculated ground state anionic binding energies (BEs) matched excellently the measured EAs. Indeed, the EAs provide a stringent test of the theory when the calculated EAs are compared with those from reliable measurements. This gave great credence to the ability of the Regge-pole methodology to extract from the anionic ground state total cross sections (TCSs), unambiguous and reliable EAs. It is noted here that theoretical TCSs and/or EAs for the fullerenes are almost non-existent, save for the small C20 and the spherically symmetric C60 and C240 fullerene molecules.
The need in organic solar cells for fullerenes with larger EAs and for the fundamental understanding of negative ion formation in low-energy electron collisions, have mostly motivated this study. The internal region of zero potential provided by the hollow cage structure of fullerenes is conducive to ground, metastable and excited anionic states formation during the low-energy electron-fullerene collisions. Entirely new in the field of electron-cluster/fullerene collisions, the Regge pole methodology, wherein is fully embedded the electron-electron correlations and the vital core-polarisation interaction, has been benchmarked on the measured EAs of atomic Au and Pt, as well as of the fullerenes C60 and C70 and used to produce the unprecedented theoretical BEs for the anions Cnˉ (n=20 through 92) that matched excellently the measured EAs. (1, 2)
Figure 1 displays the electron elastic TCSs for the relatively large C100, typical of large fullerenes. Indeed, they are characterised by ground, metastable and excited negative ion formation (1-3), represented by the dramatic sharp peaks. The hollow nature of the fullerene molecules supports the two prominent groups of the dramatically sharp resonances. The first is bounded by the ground state TCS curve (red curve), with the anionic BE located at the deepest Ramsauer-Townsend (R-T) minimum and a shape resonance near threshold.
This BE corresponds to the EA of the C100. The second group belongs to the excited states (orange, green and pink curves). Most important here is that the limiting orange curve is robust; it has been used to determine fullerene and atomic behaviour. (1) For fullerene behaviour, the ratio of the first to the second R-T minima is greater than unity, indicative of the strong polarisability of fullerenes, while for atomic behaviour, it is the opposite. The results in (1-3) and those obtained for the actinide atoms should be useful in the understanding of inter alia the recently characterised Th@C76 as well as other endohedrals and the recently measured EA of Th.
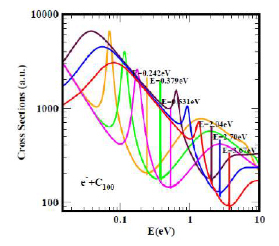
The resonance structures in the low-energy electron elastic TCSs for the fullerene molecules have led to new manifestations.
Namely, the Regge-pole calculated electron elastic TCSs for all the Cn (n=20 through 112) were found to be characterised by R-T minima, shape resonances and ground, metastable and excited anionic formation. Additionally, we investigated the size effect through the induced metastable resonances as the fullerene size varied from C20 through C112. We found that the C20 TCSs exhibited atomic behaviour while those for the C112 displayed strong departure from atomic behaviour due to the size effect; it impacts significantly the polarisation interaction inducing a series of long-lived metastable C112ˉ anions.
Surprisingly, the small C24, with its TCSs exhibiting mild atomic behaviour, has a large EA value of 3.80 eV. It is, therefore, suitable for use in countering the rate of irreversible polymer photobleaching and for resisting the fullerene degradation by the photo-oxidation mechanism in organic solar cells. The identified metastable anions could also be useful in the oxidation of methane to methanol without CO2 emission as well as serve as an inexpensive single nanocatalyst.
The rich long-lived metastable and excited states resonances, characterising the Regge-pole calculated electron elastic TCSs for fullerene molecules presented for the first time in (1, 3) support the important conclusion that the experimentally detected fullerene isomers correspond to the metastable states. (4) They certainly confirm the need to delineate and identify the resonance structures in low-energy electron collisions, since the formed anions determine the fullerene nanocatalysis mechanism.
The relatively large ground state anionic BEs found for many fullerenes should satisfy partly the requirement of employing fullerenes with high EAs (5) in order to increase the fullerene acceptor resistance to the degradation of organic solar cells by the photo-oxidation mechanism. These results should also guide both the experimental and theoretical exploration of the fullerenes, particularly the large ones. Significantly, a single large fullerene such as the C136 or even the C100 could replace the Au, Pd and Sn atoms in the catalysis of H2O2 from H2O in the experiments of Hutchings and collaborators.(6) Indeed, this could reduce the cost significantly and provide a cost-effective dynamic H2O2 nanocatalysis for water purification in the developing world. (6)
With the appropriate vertical detachment energy matching the anionic BEs of many fullerene molecules, chemical reactions could be catalysed. Additionally, many of the fullerenes could also be combined to form super multiple catalysts/sensors for various chemical reactions as well as used as multiple electron acceptors in the condensed phase. The fundamental understanding of slow electron collision processes at the atomic and nano scales leading to anionic formation will certainly impact new materials design and creation significantly.
Representing an unprecedented theoretical breakthrough in low-energy electron-cluster/fullerene collision, the robust Regge-pole approach will certainly continue to be used to identify suitable fullerenes for various applications through the EAs calculations. These EAs have now been firmed to correspond to the ground state BEs of the formed negative ions during the collisions. The strength of the methodology lies in that it requires no assistance whatsoever from either experiment or other theory to achieve the remarkable feat.
References
1. A. Z. Msezane and Z. Felfli, Chem. Phys. 503, 50 (2018).
2. Z. Felfli and A. Z. Msezane, Euro Phys. J. D 72, 78 (2018).
3. A. Z. Msezane, Z. Felfli, V.R. Shaginyan and M. Ya. Amusia, Int.
J. of Current Advanced Research, 6(12) 8503 (2017).
4. L. Kronik, et al. Nature Mats. 1, 49 (2002).
5. E. T. Hoke, et al. Adv. Energy Mat. 2, 1351 (2012).
6. S. J. Freakley, et al. Science 351, 959 (2016)
*Please note: This is a commercial profile











