Alexander Gebauer, the Chief Executive Officer at Secarna Pharmaceuticals GmbH & Co. KG looks at a new approach to solve unmet medical needs with antisense therapies
Despite major therapeutic advances, there is a variety of diseases that are still not adequately addressed by traditional approaches such as small molecules or antibodies. For those diseases with clearly defined targets, one could expect a speedy development of effective treatment options. However, this is not always the case: due to their unique characteristics, some genes have been deemed challenging to be targeted with traditional therapeutic modalities.
Antisense therapies emerged in the late 1980s and have evolved to fill the treatment gap of various diseases and expand the scope of targetable genes, offering the potential to address, or even cure, a broad range of diseases caused or driven by aberrant gene expression. (1)
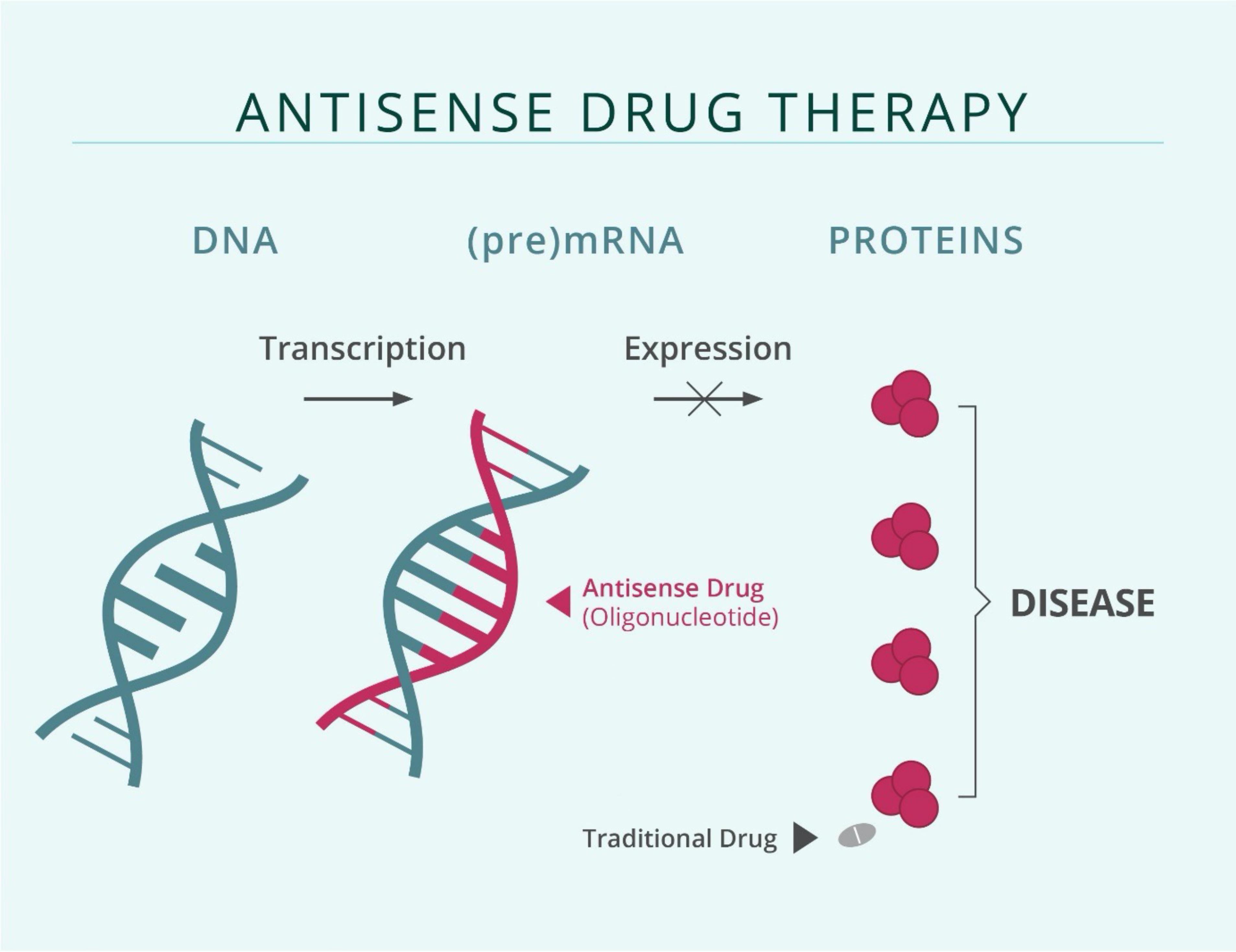
The mechanism of action of antisense oligonucleotide (ASO)-based therapies that are designed to suppress gene expression
The genetic information of a cell is stored in the form of a double-stranded DNA in the nucleus. In a process known as transcription, genetic information stored in the DNA is transcribed into RNA, having either regulatory functions or coding for proteins. These molecules, when aberrantly expressed, can give way to the development or progression of many different diseases. Developing therapeutics that target gene expression at the RNA-level provides the opportunity to use nucleic acid sequence specificity to approach targets that are challenging to drug at the protein level.
Antisense oligonucleotides (ASOs) are synthetic, chemically modified, short, single-stranded DNA sequences complementary to their target RNA and are designed to specifically bind only to their target. Chemical modifications increase stability against degradation, as well as cellular uptake and binding affinity to the target, and reduce unwanted side effects. After the ASO binds to the target RNA, the target RNA is degraded by cellular mechanisms and cannot exert its regulatory functions or be translated into proteins that promote disease development and progression.
Why are ASOs superior to other forms of treatment?
ASOs are clearly distinguished from traditional therapeutic approaches – with obvious potential advantages. Conventional small-molecule drug development requires a strong effort to screen and optimize potential candidate structures. Oligonucleotide drug candidates, on the other hand, can be rationally designed based on sequence information and rapidly screened. Compared with small- molecule drugs, substantially lower numbers of compounds need to be tested to obtain potent, specific and safe compounds. Preventing the expression of disease-modulating proteins by ASOs offers some clear advantages compared to modulating their activity with small molecules or antibodies after their expression, especially for targets having different functions exerted by different domains.
The success of therapeutics such as inotersen for the treatment of hereditary transthyretin amyloidosis (ATTR), volanesorsen for treatment of familial chylomicronaemia syndrome (FCS), and nusinersen, the first approved drug for the treatment of spinal muscular atrophy (SMA), have shown that there are clearly treatment gaps that can be filled by antisense therapeutics (2). The steady stream of transactions for antisense, siRNA and mRNA therapeutics shows that the industry is taking these modalities seriously as alternatives to traditional small molecules and antibodies.
Advances in RNA-targeting platforms and technologies, such as advanced sequencing technologies, bioinformatics, structural biology, screening methodologies, and chemistry are converging to inform the rational design and identification of novel therapeutics.
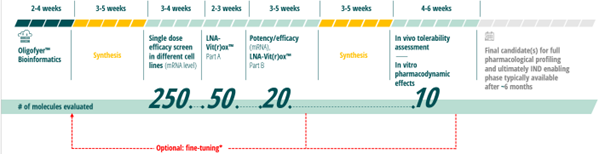
Secarna’s 3rd generation proprietary LNAplus™ ASO platform enables rapid development candidate generation for highly specific, safe and efficacious therapies for patients in need Secarna Pharmaceuticals was founded in 2015, in Marburg, Germany, based on the great potential offered by RNA- targeting platforms. The Company developed a 3rd generation antisense therapies platform, LNAplus™, that encompasses all aspects of drug discovery and pre-clinical development, including powerful proprietary Oligofyer™ bioinformatics. Oligofyer™ is able to fully screen all relevant databases and analyze the target RNA, providing sufficient information to select the most specific ASO drug candidates. Furthermore, these sequences are filtered, and only those with superior probability for efficacy and tolerability are synthesized and tested.
Since its inception, Secarna has become a global leader in discovering and developing ASOs. The Company’s 3rd generation chemistry has major advantages compared to previous generations of ASOs, including significantly improved potency and reduced unwanted stimulation of the immune system, ensuring potent activity and good tolerability. Unlike siRNA or older ASO generations, they do not require delivery reagents for potent target knockdown in a variety of organs and tissues. Still, they are amenable to conjugation with different ligands such as GalNAc to allow for cell-specific delivery, if required.
After ASOs are synthesized, they are screened under physiological screening conditions. Promising candidates are subjected to a number of tests by the LNA Vit(r)ox™ test system, which recognizes and eliminates compounds with safety liabilities before the most promising candidates are tested for functional activity in target-specific functional assays.
LNAplus™ enables rapid candidate generation with lead compound identification in only 24 weeks by virtue of a highly streamlined process for achieving a clinical development candidate.
A rich pipeline of promising pre-clinical ASO programs offers attractive licensing opportunities for pharma partners
Secarna has a focused in-house pipeline of advanced pre-clinical ASO programs in the field of immune- oncology as well as inflammatory and fibrotic diseases, concentrating on targets that are either considered as challenging to target by conventional approaches or where ASOs have clear advantages over other modalities due to their mechanism of action, biodistribution, or pharmacokinetics.
LNAplus™-derived ASOs have been validated by in-house as well as successful pharma-partnered projects, including large programs with Evotec and Denali. Another example of Secarna’s success is the Company’s collaboration with Lipigon, under which the first ASO derived from the LNAplus™ platform was advanced into the clinic in 2022.
Secarna: The leading European independent ASO company with the vision to broaden the universe of druggable targets
To date, nine ASOs have been approved by the US Food and Drug Administration (FDA), and the majority of them have achieved marketing authorization in the last five years(3). Several other ASOs, including Secarna-discovered Lipisense from the Swedish company Lipigon, are currently in clinical trials to treat a variety of indications, including neurological, cardiovascular, hepatic, and ophthalmic disorders.
Secarna seeks to establish ASO therapeutics as the third pillar in drug development, next to small molecules and antibodies, by becoming the partner-of-choice for ASO drug discovery and development programs and to form strategic partnerships fully leveraging its collaborators’ as well as the Company’s own strengths.
References

This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International.