University of Perugia’s Ursula Grohmann and Antonio Macchiarulo share the work of the DIDO project in understanding cancer and autoimmune disease therapies
As proposed by Burnet and Thomas in their theory of ‘cancer immune-surveillance’ more than 50 years ago, our immune system is endowed with the capacity to identify and eliminate tumour cells. However, immune responses must be properly controlled in order to prevent the destruction of organism’s cells and tissues, as it occurs in autoimmune diseases. Such physiological control mechanisms, or ‘brakes’ of the immune system, are commonly known as checkpoints. Whereas defects in check-points can lead to autoimmune diseases, cancer cells have learnt how to successfully exploit such brakes in order to escape from an immune attack. The generation of check-point inhibitors, i.e., drugs releasing critical brakes off the immune system, has revolutionised the therapeutic treatment of certain cancers, such as melanoma, kidney and small cell lung tumours. Nevertheless, the low percentage of responsive patients, as well as the limited number of tractable tumours would require innovative inputs to the check-point inhibitor field. On the other hand, no therapeutic approach has been developed so far on the basis of a potentiation of check-point activity in autoimmune diseases.
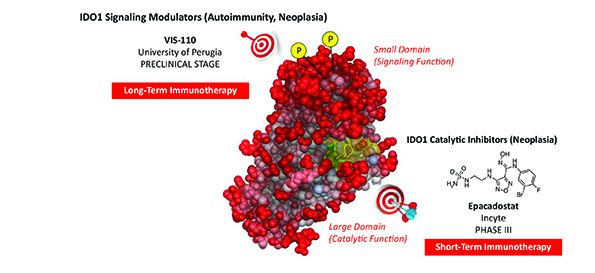
An important mechanism of immune escape in tumours involves the metabolism of tryptophan (Trp), the rarest essential amino acid found in food. More than 90% of Trp entered with diet is metabolised via a pathway that produces several biologically active molecules, collectively known as kynurenines. Both Trp degradation and the production of kynurenines are involved in the brake effects of Trp metabolism on the immune system and, in this regard, the main enzyme involved is indoleamine 2,3-dioxygenase 1 (IDO1). The highest expression of IDO1 can be found physiologically in dendritic cells (DCs), sentinels of the immune system that instruct T lymphocytes to either mount an effective immune response to destroy the foreign invaders or tolerate (not respond to) the antigens. A deficiency in IDO1 activity, particularly in DCs, has been observed in several experimental models, as well as human diseases of chronic inflammation and autoimmunity. On the other hand, tumour-bearing animals, as well as neoplastic patients, are often characterised by an overexpression of the IDO1 enzyme, thus dampening anti-tumour immunity. Hence, IDO1 may represent an additional check-point molecule and, indeed, a combination of pembrolizumab (a ‘classical’ check-point inhibitor) and epacadostat, (a potent inhibitor of IDO1 catalytic activity) has recently reached phase III in clinical trials (ClinicalTrials.gov: NCT02752074).
In 2011 (1), we discovered that IDO1 is not just an enzyme metabolising Trp, but once phosphorylated by other enzymes, can trigger a signalling pathway that completely reprograms an immunostimulatory DC into a tolerogenic cell. Notably, we also discovered that, whereas the tolerogenic effects of IDO1 as an enzyme degrading Trp are transient, a DC reprogrammed via the IDO1 signalling pathway will be capable to maintain tolerance and thus keep in check auto- and also anti-tumour immunity over the long-term. Thus our data suggested that the positive or negative modulation of IDO1 signalling by small molecules rather than catalytic activity could be more effective in the control of two chronic diseases such as autoimmunity and neoplasia, respectively (Figure 1).
The DIDO Project
DIDO is a 5-year project funded by the European Research Council and led by Prof. Ursula Grohmann (principal investigator) and Prof. Antonio Macchiarulo (team member). It started in early February 2014 and is due to end in late January 2019. The aim of DIDO is to identify small molecules capable of acting over the long-term by either favouring (thus useful in the therapy of chronic inflammation and autoimmune disease) or inhibiting (useful in neoplasia) the IDO1 signalling. To this purpose, the expertise in pharmacology/Immunology/biology (PI’s group) has been integrated with the expertise in medicinal chemistry/molecular and computational modelling (team member’s group) to develop such innovative drugs.
In the first 30 months of the project, we established a novel, integrated method capable of screening hundreds of compounds with the potential of modulating IDO1 signalling. We screened more than 200 structurally diverse compounds selected from a structure-based virtual screening of ~600.000 small molecules endowed with drug-like or lead-like properties. Of these, dozen compounds were so far proven to be novel, potent IDO1 catalytic inhibitors. We also identified chemical structures capable of modulating the signalling and not catalytic activity of IDO1. Specifically, one of these, VIS-110, did not show significant inhibitory effects on IDO1 catalysis but did promote the IDO1 signalling and, when used in DC cultures, conferred long-term tolerogenic properties capable of blocking the presentation of an autoantigen in vivo in an IDO1-dependent fashion. Moreover, the same molecule was capable to protect mice from experimental autoimmune encephalomyelitis, a preclinical model of human multiple sclerosis.
Finally, we unexpectedly discovered an IDO1 catalytic enhancer, i.e., VIS-119, capable of increasing the affinity of IDO1 for its substrate Trp in both cell and biochemical (using a recombinant IDO1 protein) assays. VIS-119 was also effective in protecting mice from the development of experimental autoimmune encephalomyelitis. Very recently, we have also identified an additional novel method that allows for the identification of inhibitors of IDO1 signalling. All selected molecules are currently subjected to studies of structure activity relationships, early ADME profiling, pharmacokinetics, toxicity and efficacy in tumour models and additional experimental disease models of autoimmunity and also allergy.
The DIDO project thus appears to have great potential to generate highly innovative drugs for the treatment of both autoimmunity and neoplasia, two wide disease classes so different in their pathologic nature.
References
1 Pallotta, M.T. et al. Indoleamine 2, 3-dioxygenase is a signalling protein in long-term tolerance by dendritic cells. Nat. Immunol. 12 (9), 870-878 (2011).
Ursula Grohmann, P.D
Full professor of pharmacology
Department of Experimental Medicine
ursula.grohmann@unipg.it
Antonio Macchiarulo, PhD
Associate professor of medicinal chemistry
Department of Pharmaceutical Sciences
antonio.macchiarulo@unipg.it
University of Perugia, Perugia, Italy
Tel: +39 075 585 8240
Please note: this is a commercial profile