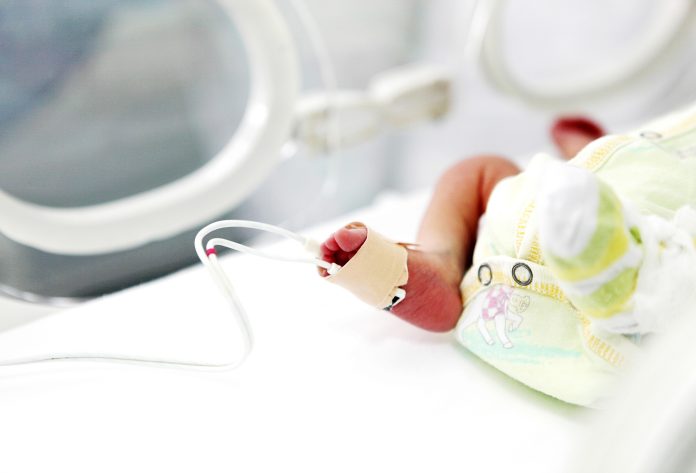
The NHS will offer a long-acting antibody injection to protect thousands of premature and vulnerable babies from respiratory syncytial virus (RSV) starting this autumn
Thousands of premature and vulnerable babies across England are set to receive enhanced protection from respiratory syncytial virus (RSV) as the NHS rolls out a new long-acting antibody injection. The initiative, launching this autumn, marks a significant step forward in preventing severe illness among at-risk infants during the winter months.
Protecting 7,000 high-risk infants
RSV, or respiratory syncytial virus, is a common virus that infects 90% of children by the age of two, and is one of the leading causes of hospitalisation. The infection can develop into life-threatening pneumonia and infant bronchiolitis. Data shows that babies born prematurely are three times more likely to need hospital admission due to RSV, and ten times more likely to require intensive care, compared to full-term babies.
NHS England is rolling out a drug called nirsevimab from late September to protect against RSV to around 7,000 high-risk infants and babies born before 32 weeks this year, helping vulnerable infants avoid hospitalisation.
Nirsevimab offers 80% protection against RSV
Nirsevimab offers six months’ protection in a single dose and will replace monthly injections of palivizumab previously provided to a smaller number of vulnerable babies. Clinical trial data indicate that palivizumab provides around 55% protection while nirsevimab offers more than 80% protection against the virus.
In addition, nirsevimab will be offered seasonally to eligible high-risk infants and young children with complex heart, lung or weakened immune system conditions.
Dr Claire Fuller, Co-National Medical Director for NHS England, said: “This injection will provide premature babies with a protective bubble against RSV just in time for the colder months.
“It will offer a long-lasting defence, helping to avoid unnecessary hospitalisations and serious illness, giving babies the best possible start in life and shielding them from harm.”
Nirsevimab works differently from the RSV vaccine offered to pregnant women, boosting the mother’s immune system to create extra protective antibodies that are passed to the baby in the womb, providing protection from birth. However, babies born before 32 weeks have limited or no protection from vaccinations that pregnant women receive at the recommended time, around 28 weeks.
John Stewart, Director of Specialised Commissioning at NHS England, said: “For babies born very prematurely, the risk of contracting RSV in their first winter is high and extremely serious.
“This new medication will provide vulnerable infants with their suit of armour that protects against what can be a life-threatening infection.
“I am delighted that we will be able to offer nirsevimab to protect these babies from this winter onwards, and I’m extremely grateful to colleagues in specialist neonatal clinics across the country who will deliver this life-saving service.”
Minister for Public Health and Prevention Ashley Dalton said: “This is excellent news for thousands of vulnerable babies and their families. By rolling out nirsevimab this September, we’re ensuring that our most at-risk infants, including those who are born too early to benefit from maternal vaccination, are protected in the best possible way.
“This rollout is a prime example of how this government is shifting the focus from sickness to prevention, as part of our 10 Year Health Plan.”
Dr Conall Watson, consultant epidemiologist at the UK Health Security Agency, said: “Having an RSV vaccine from week 28 in every pregnancy is key to protecting your baby against RSV bronchiolitis. Most RSV hospital admissions in babies can be prevented by vaccination.
“This new NHS nirsevimab immunisation programme will offer critical protection for very premature babies born before mum’s antenatal vaccination can protect them.
“UKHSA has been working closely with JCVI and the NHS to provide evidence in support of this programme, and we are delighted to see this launch ahead of winter 2025.”