Signal Peptide Peptidase-Like proteases as promising targets to fight immunological diseases – Prof. Regina Fluhrer from LMU & DZNE and Professor Bernd Schröder from TU Dresden discuss how intramembrane proteases contribute to the development of immune cells
Signal Peptide Peptidase-Like (SPPL) proteases are intramembrane proteases and homologues of Signal Peptide Peptidase (SPP) the founding member of the SPP/SPPL proteases family. In humans, the SPP/SPPL family comprises five members: SPP, SPPL2a, SPPL2b, SPPL2c and SPPL3. Although they are summed in one family, their localisation in the cell and their physiological functions differ greatly.
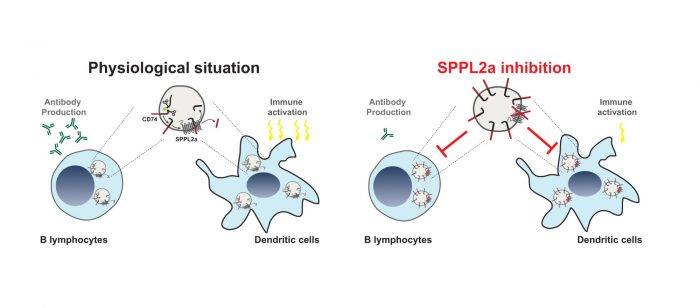
SPPL2a localises to membranes of lysosomes, an intracellular compartment involved in degradation of proteins and other macromolecules. Although the complete substrate spectrum of SPPL2a is still far from being known, CD74 a protein of particular importance in immune cells has been recently demonstrated to be cleaved by SPPL2a in its membrane spanning domain. A specific step in the degradation of the CD74 protein requires intramembrane proteolysis by SPPL2a. Therefore, in absence of the protease a small, but still membrane-embedded fragment of CD74 massively accumulates in B cells and dendritic cells and disturbs their development and function. While dendritic cells are important for recognition and fighting invading pathogens B cells produce antibodies targeting the “intruders” which is a major mechanism to clear infections. Loss of SPPL2a activity in mice results in an impairment of both cell types and in humans, it causes an increased susceptibility to infections with mycobacteria. The reduced ability of humans with mutations in the SPPL2a gene to fight mycobacterial infections like tuberculosis can be explained by a loss of specific dendritic cells, which are required to trigger immune responses against these pathogens. Thus, presence of SPPL2a and its ability to degrade CD74 represents an important checkpoint in B cells and dendritic cells and may allow to deplete them in patients with SPPL2a specific inhibitors. This could be beneficial in different disease contexts like multiple sclerosis or systemic lupus erythematosus and represents an interesting novel concept that may be tested for potential treatment strategies in the future.
SPPL3, another member of the SPP/SPPL family, localizes to membranes delimiting the Golgi apparatus, a cellular compartment that is critical for protein maturation and sorting. It is expressed in almost every human cell and regulates the glycan composition of membrane proteins (see Special Report in January 2019). In addition, it is crucial for normal development of Natural Killer cells (NK cells). NK cells are part of the innate immune system and critical for tumor surveillance as well as clearance of virally infected cells. After development in the bone marrow, NK cells migrate and populate all organs of the body where they undergo final maturations steps and become fully active “guardians”. Deficiency of SPPL3 in mice results in impaired maturation of NK cells and reduced clearance of tumor cells. This makes SPPL3 an interesting player in the immune system and in tumor defence. However, before SPPL3 is considered as a therapeutic target, it needs to be further investigated if it is indeed dysregulated in immune deficiencies or malignant tumors.
These examples illustrate that SPPL proteases are involved in development and function of the immune system, but also elucidate that we are far from understanding the whole context in detail. Thus it is of critical importance to invest more time and resources in understanding the role of intramembrane proteases in the immune system to utilize them as valuable drug targets in the future.
Further reading:
Voss M, Schröder B, Fluhrer R. 2013. Mechanism, specificity, and physiology of signal peptide peptidase (SPP) and SPP-like proteases. Biochim Biophys Acta. 2013 Dec;1828(12):2828-39.
Mentrup T, Fluhrer R, Schröder B. 2017. Latest emerging functions of SPP/SPPL intramembrane proteases. Eur J Cell Biol. 96(5):372-382.
Schneppenheim et al., The intramembrane protease SPPL2a promotes B cell development and controls endosomal traffic by cleavage of the invariant chain. J Exp Med 2013, 210, 41-58.
Hamblet et al., NK Cell Maturation and Cytotoxicity Are Controlled by the Intramembrane Aspartyl Protease SPPL3. J Immunol. 2016 Mar 15;196(6):2614-26.




Institute of Physiological Chemistry at the Technische Universität Dresden, Dresden, Germany









