New research reveals that a routine blood test measuring insulin resistance via the TyG index may identify individuals with early Alzheimer’s who are four times more likely to experience rapid cognitive decline
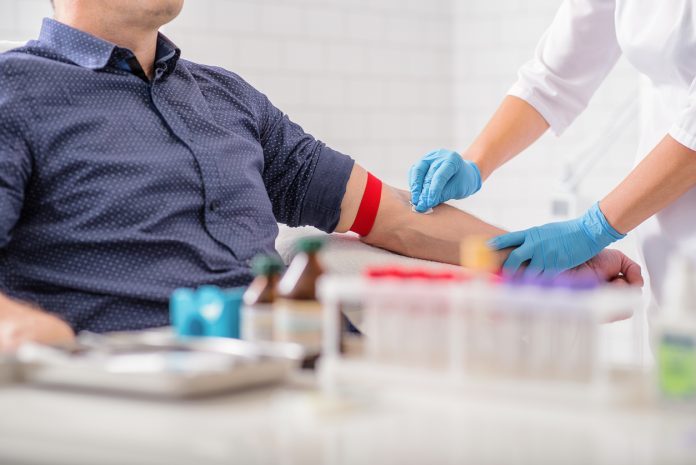
A routine, low-cost blood test could offer new insight into how quickly Alzheimer’s disease progresses in its early stages. Researchers presented striking findings at the European Academy of Neurology (EAN) Congress 2025, suggesting that a simple metabolic marker may help identify patients at a greater risk of rapid cognitive decline. With early intervention key to managing Alzheimer’s, the study not only opens the door to more targeted and timely care but also instils hope for patients and their families, without the need for expensive or invasive diagnostics.
Insulin resistance is linked to Alzheimer’s disease
Insulin resistance may do more than trigger Alzheimer’s; it could speed it up. While previously linked to the onset of the disease, its role in progression remained unclear. The study, led by neurologists at the University of Brescia, aimed to shed light upon this research area by focusing on insulin’s impact during the prodromal mild cognitive impairment (MCI) stage, when patients follow highly variable trajectories.
The researchers utilised the TyG index, a low-cost, routinely available surrogate for insulin resistance, to investigate whether metabolic dysfunction could help predict the rate of cognitive decline following diagnosis. This simple and accessible test could potentially revolutionise the way we approach Alzheimer’s disease, providing a reliable tool for predicting cognitive decline.
A simple test identifies vulnerable patients
The team of neurologists reviewed the records of 315 non-diabetic patients with cognitive deficits, including 200 with biologically confirmed Alzheimer’s disease. The patients had their insulin resistance assessed using the TyG index, and a clinical follow-up was conducted over three years.
The researchers were divided according to the TyG index, and those in the highest third of the Mild Cognitive Impairment AD subgroup deteriorated significantly more quickly than their lower-TyG peers, losing more than 2.5 points on the Mini-Mental State Examination (MMSE) per year. The MMSE is a widely used test for assessing cognitive function, and a decline of this magnitude is considered significant in the context of Alzheimer’s disease (hazard ratio 4.08, 95% CI 1.06–15.73). No such link appeared in the non-AD cohort.
“Once mild cognitive impairment is diagnosed, families always ask how fast it will progress”, said lead investigator Dr Bianca Gumina. “Our data show that a simple metabolic marker available in every hospital laboratory can help identify more vulnerable subjects who may be suitable candidates for targeted therapy or specific intervention strategies.” This potential for targeted interventions could empower healthcare professionals and patients alike in the fight against Alzheimer’s.
The research suggests that insulin resistance may impair brain glucose use, promote amyloid buildup, disrupt the blood-brain barrier, and fuel inflammation, key drivers of a faster decline.
“We were surprised to see the effect only in the Alzheimer’s spectrum and not in other neurodegenerative diseases”, Dr Gumina noted. “It suggests a disease-specific vulnerability to metabolic stress during the prodromal window, when interventions may still change the trajectory.”
The research team, led by Professor Padovani and Professor Pilotto, found that high TyG was also associated with blood-brain barrier disruption and cardiovascular risk factors. Yet, it showed no interaction with the APOE ε4 genotype, indicating that metabolic and genetic risks may act through distinct pathways.
Identifying high-TyG patients could refine enrollment for anti-amyloid or anti-tau trials and prompt earlier lifestyle or pharmacological measures to improve insulin sensitivity. The researchers are currently investigating whether TyG levels can also be tracked using neuroimaging biomarkers to aid in earlier detection and stratification. If successful, this could lead to a more personalised approach to Alzheimer’s disease management, with treatments tailored to the individual’s metabolic profile and disease progression.
“If targeting metabolism can delay progression, we will have a readily modifiable target that works alongside emerging disease-modifying drugs”, concluded Dr Gumina.