Here, Laser Ablation in Liquid (LAL) is explored as means to understand the shapes and sizes of nanoparticles
The unique optical, electronic, and catalytic properties of metal nanoparticles as compared to their bulk metal counterparts depend strongly on the nanoparticles’ sizes and shapes. For instance, nanoparticles of noble metals such as gold and silver exhibit a localized surface plasmon resonance (LSPR) arising from the collective oscillations of the free electron cloud, which is sensitive to the nanoparticle size, shape, and composition. As a result, controlling the size and shape of gold and silver nanoparticles makes it possible to tune their optoelectronic properties for applications such as photothermal therapy, biosensing, and photocatalysis.
Decades of research into wet-chemical synthetic routes to noble metal nanoparticles have led to the discovery of nanoparticle morphologies ranging from atomically precise gold nanoclusters with as few as 13 atoms to larger spherical, cubic, octahedral, and triangular prism gold and silver nanostructures. This exquisite level of control over nanoparticle morphologies is typically exerted through a combination of two strategies: controlling the nanoparticle growth kinetics through tuning parameters such as reducing agent concentration, temperature, solvent, and salt additives; and adding organic surfactants or polymers that attach to the nanoparticle surfaces as ligands and prevent excessive growth. However, these strategies often require excess quantities of reducing agents and surfactants, which produces significant amounts of chemical waste. Moreover, organic ligands must be removed for certain applications due to their toxicity or because they block catalytically active sites.
Laser Ablation in Liquid (LAL) methods have emerged as promising routes to colloidal nanoparticles that have myriad advantages over wet-chemical methods. For instance, laser processing can be carried out under ambient conditions and requires no toxic chemical reducing agents, thus minimising chemical waste generation. Moreover, laser processing produces colloidally stable noble metal nanoparticles without organic capping ligands, which yields uncoated “naked” nanoparticles particularly suited to catalytic and biomedical applications. However, the absence of capping agents makes producing narrow nanoparticle size distributions with LAL challenging. For instance, gold nanoparticles (AuNPs) produced by “top-down” laser ablation of a gold target in pure water typically exhibit broad size distributions of spherical particles ranging from ~5 – 100 nm or larger. Our laboratory’s work found that “bottom-up” laser-induced reduction of aqueous tetrachloroaurate salt precursor to AuNPs also produces broad particle size distributions from ~3 – 30 nm or larger, depending on the reaction conditions1-4.
From nearly the beginning of top-down LAL investigations in the early 2000’s, investigators have added organic capping agents used in wet-chemical synthesis such as sodium dodecyl sulfate (SDS) or poly(ethylene glycol) (PEG) to the water solvent prior to immersion of the gold target. This strategy effectively produces predominantly sub-10 nm AuNPs from top-down LAL. For bottom-up AuNP synthesis via tetrachloroaurate reduction, we found that adding PEG to the solution forms AuNPs as small as 3.9±0.7 nm1, but producing such small and monodisperse AuNPs without PEG had remained elusive2,3.
To control AuNP sizes in bottom-up tetrachloroaurate reduction without capping agents, we turned to the other popular wet-chemical strategy: controlling the reaction kinetics by manipulating the availability of reducing agents in solution. The laser-induced decomposition of water produces two important reactive species that can reduce the tetrachloroaurate ion: hydrated electrons and hydrogen peroxide, which is formed from the recombination of hydroxyl radicals. Electrons are particularly strong reducing agents, and our laboratory found that hydrogen peroxide accelerates the reduction of tetrachloroaurate when AuNPs are already present through an autocatalytic growth mechanism1-4.
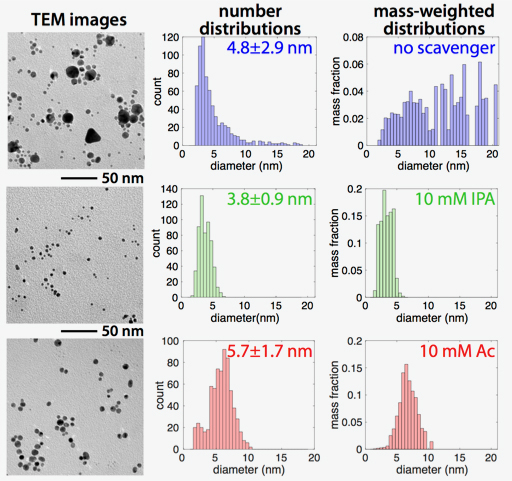
We reasoned that hydrogen peroxide-induced autocatalytic AuNP growth was to blame for the difficulty in controlling AuNP size distributions. To block this detrimental action of hydrogen peroxide, we added the well-known hydroxyl radical scavengers isopropyl alcohol (IPA) and sodium acetate (Ac) to the tetrachloroaurate solution to prevent hydrogen peroxide formation. With sufficient amounts of added scavengers, the autocatalytic AuNP growth was significantly slowed and monodisperse AuNPs as small as 3.8±0.9 nm were produced (Figure 1)4. In particular, the addition of scavengers completely eliminated the formation of nanoparticles larger than 10 nm, as compared to the no-scavenger product that exhibits more than 50% of the Au mass in >10 nm particles.
These results demonstrate that controlling the availability of reducing agents produced by laser-induced solvent decomposition enables exceptional control over both the reaction kinetics and the resulting particle size distributions in bottom-up LAL. Combined with earlier results demonstrating the efficacy of capping agents in controlling nanoparticle sizes both for top-down and bottom-up LAL, it is evident that applying the lessons of wet-chemical synthesis can significantly improve the quality of LAL-synthesized nanoparticles. Our group is pursuing additional investigations into understanding and manipulating reaction kinetics in bottom-up LAL that will equip the LAL community with tools needed to exert improved control over nanoparticle sizes, making them suitable for practical applications.
References
1 B. Tangeysh, K. M. Tibbetts, J. H. Odhner, B. B. Wayland, and R. J. Levis, J. Phys. Chem. C 117 18719-18727 (2013) DOI: 10.1021/jp4056494
2 K. M. Tibbetts, B. Tangeysh, J. H. Odhner, and R. J. Levis, J. Phys. Chem. A 120 3562-3569 (2016) DOI: 10.1021/acs.jpca.6b03163
3 V. K. Meader, M. G. John, C. J. Rodrigues, and K. M. Tibbetts, J. Phys. Chem. A 121 6742-6754 (2017) DOI: 10.1021/acs.jpca.7b05370
4 L. M. Frias Batista, V. K. Meader, K. Romero, K. Kunzler, F. Kabir, A. Bullock, and K. M. Tibbetts, J. Phys. Chem. B 123 7204-7213 (2019) DOI: 10.1021/acs.jpcb.9b04643
Please note: This is a commercial profile