Katharine Moore Tibbetts, Assistant Professor at Virginia Commonwealth University, shares with us her expertise on advances in engineering functional nanomaterials
Nanomaterials have well-defined structures on length scales smaller than 100 nm (1,000,000,000 nm = 1 m), close to the size of individual atoms and molecules. These small sizes impart unique optical, electronic and catalytic properties that cannot be obtained in bulk materials.
In recent years, advances in engineering functional nanomaterials have revolutionised applications from cancer therapy and drug delivery to industrial catalysis and solar fuel generation. Nanomaterials are, therefore, enabling 21st-century technologies to meet major challenges to society such as sustainable energy development.
While immense progress in synthetic methods over the past three decades has made it possible to create tailored nanomaterials with well-defined sizes and compositions, the most commonly used wet-chemical synthetic methods suffer from two major drawbacks.
First, they typically require excessive quantities of toxic chemicals that generate large amounts of waste. For instance, the standard procedure for making ultrasmall <2 nm gold nanoparticles requires a 10-fold excess of reducing agent and at least 3-fold excess of capping ligand. Second, the capping ligands that are needed to control nanoparticle sizes can hinder the use of these nanoparticles in applications.
For instance, cytotoxic ligands must be removed from metal nanoparticles prior to use in biomedical applications and organic ligands can block access to catalytically active surface sites in metal nanoparticle catalysts. As a result, “naked” nanoparticles with no capping ligands are highly desirable for these and other applications.
Laser ablation in liquid (LAL) has recently emerged as a versatile synthetic route to a variety of nanomaterials that overcomes the major drawbacks of wet-chemical synthesis. Instead of requiring a complex mixture of toxic chemicals, LAL can produce nanomaterials using only water and solid or powdered target material. The lack of toxic chemical use and waste generation means that LAL methods satisfy the major principles of “green chemistry” and can become a platform for environmentally sustainable nanotechnology development. Moreover, nanoparticles synthesised by LAL often do not require capping ligands because they emerge electrostatically stabilised. As a result, LAL is a powerful method for producing naked nanoparticles uniquely suited to biomedical and catalysis applications.
These advantages of LAL coupled to advances in commercial pulsed laser technology have generated surging interest in LAL for nanomaterial synthesis. This interest is captured by the 30-fold increase in citations from an ISI Web of Knowledge search of “laser synthesis nanoparticles” from 563 in 2003 to 16,925 in 2018. LAL-synthesised nanomaterials show promise in applications such as electrochemical water-splitting, photocatalytic hydrogen generation and photothermal cancer therapy. While LAL manufacturing of nanomaterials at industrial scales is likely years away, grammes per hour production rates are now attainable with advanced high-repetition-rate lasers.
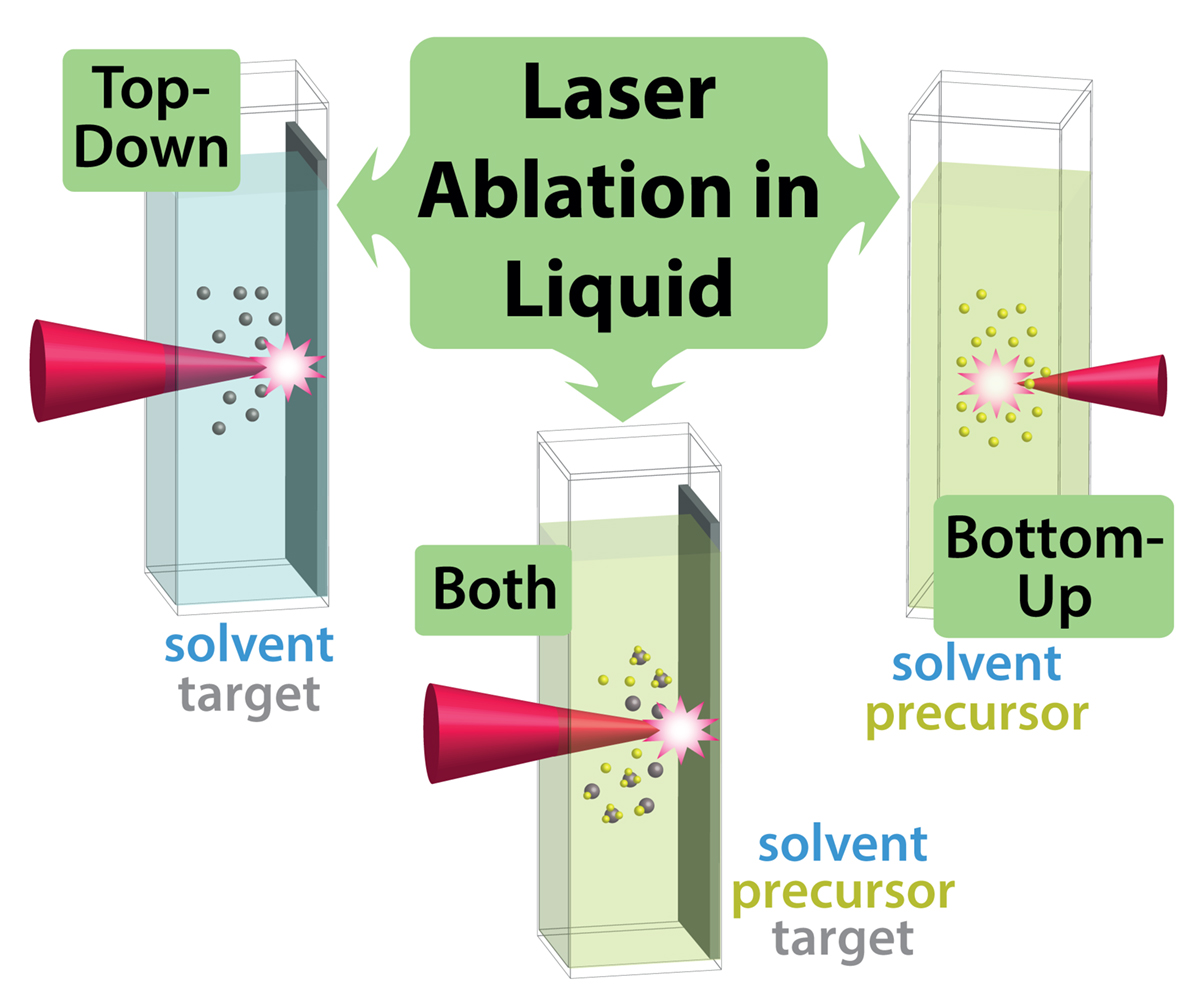
In LAL, a high-power pulsed laser is focused into a liquid medium or onto a solid-liquid interface (Figure 1). LAL encompasses both “top-down” and “bottom-up”, as well as combined approaches to nanomaterial synthesis. In top-down methods, an immersed solid or powder of the target material produces nanomaterials in the surrounding solution as atoms and clusters are blasted off of the surface or powder particles are fragmented in the laser focus.
In bottom-up methods, a precursor such as a metal salt dissolved in solution produces nanomaterials through photochemical reactions of the precursor and solvent initiated by laser irradiation. Combined approaches involve immersion of a solid or powder in a precursor solution to produce composite nanomaterials from ablated material reacting with the precursor and solvent.
The top-down approach is by far the most widely used in the LAL community due to the low cost of solid materials relative to molecular precursors, simplicity of performing synthesis reactions and ability to manufacture large quantities of nanomaterial products. However, a significant drawback of top-down approaches is the often limited control over the size distributions of the resulting nanoparticles. For instance, gold nanoparticles have been synthesised by top-down ablation of gold foil for at least two decades, but even recent studies typically report asymmetric size distributions containing large >20 nm particles despite a mean particle size of 5 nm.
For applications where tight particle size distributions are needed, bottom-up LAL synthesis represents a promising alternative. For instance, our laboratory recently reported uniform 5-nm gold nanoparticles using bottom-up photoreduction of the tetrachloroaurate salt in water using a commercial 532 nm Nd:YAG laser (Figure 2) (1).
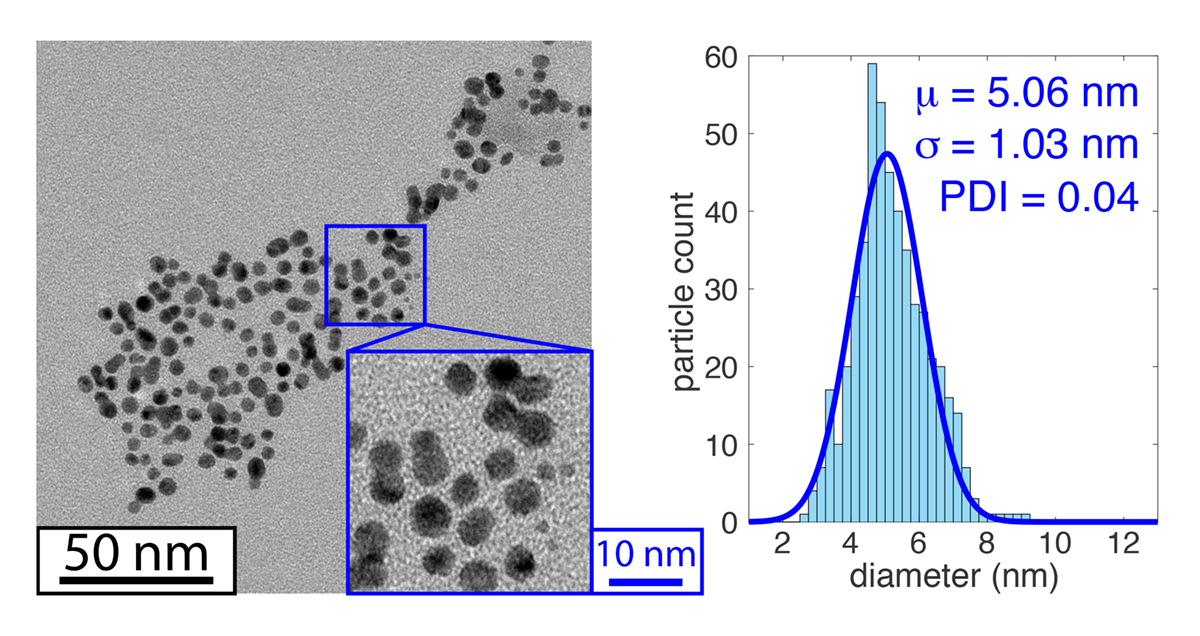
While bottom-up LAL synthesis has received less attention than the common top-down methods, it represents a potential platform for tailoring photochemical reactions to attain exquisite control over the sizes and compositions of nanomaterials produced with LAL. Achieving this potential will require advances in understanding the photochemical reaction pathways that convert precursors in solution to nanomaterials, which is a primary goal of our laboratory’s research.
References
(1) C. J. Rodrigues, J. A. Bobb, M. G. John, S. P. Fisenko, M. S.
El-Shall, and K. M. Tibbetts, Phys. Chem. Chem. Phys. 20 28465-28475 (2018).
Katharine Moore Tibbetts
Assistant Professor
Virginia Commonwealth University
Tel: +1 804 828 7515
kmtibbetts@vcu.edu
https://chemistry.vcu.edu/people/faculty/tibbetts.html
*Please note: This is a commercial profile











