Chester Medical School explores the pathogenesis of Diabetes Mellitus Type 2 (T2DM) with novel approaches
Diabetes Mellitus Type 2 (T2DM) remains a major public health problem with financial implications for healthcare systems worldwide. According to Diabetes UK, 3.7 million adults were diagnosed with diabetes mellitus and 12.3 million were at risk of developing the disease in 2017. The cost to the NHS is an estimated £1.5 million an hour – equating to about 14 billion a year.
A report by the Health and Social Care Information Centre has also shown in the eight years between 2005/06 to 2013/14, the cost of drugs and treatment of T2DM had risen by 56.3% 1. It is recognised that a major driver for T2DM is obesity-induced insulin resistance. Insulin resistance is a condition where the insulin produced by the pancreas is unable to work properly resulting in high blood glucose and T2DM. The mechanism by which obesity induces insulin resistance signals the induction of T2DM has not been fully elucidated. Research programmes at Chester Medical School aim to shed light on potential mechanisms by which obesity triggers the pathogenesis of insulin resistance T2DM.
How obesity causes insulin resistance
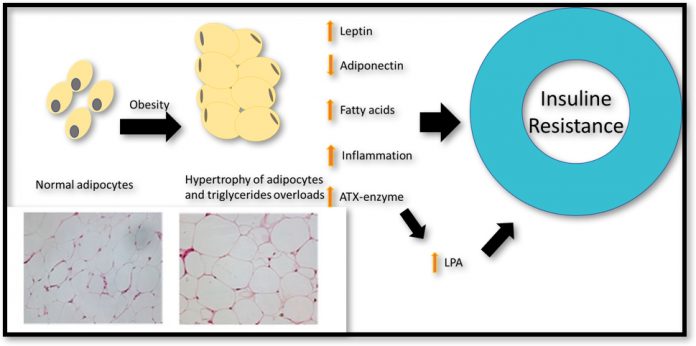
Obesity is essentially the abnormal accumulation of body fat in a process involving the increase of fat cells size and number – known as hypertrophy and hyperplasia. During hypertrophy and hyperplasia fats cells release bioactive adipokines and enzymes that regulate the uptake of triglycerides in the blood. Leptin and adiponectin are major adipokines, which are released from fat tissue in the abdominal area and have an essential role in regulating blood sugar level. Leptin increases fatty acid oxidation and reduces blood sugar and body fat.
Adiponectin, however, plays an essential role in stimulating insulin sensitivity and its level is decreased by obesity. Conversely, the level of leptin has been shown to increase in obesity due to leptin resistance. Leptin normally signals the brain to quench hunger sensation when enough energy is stored in the fat cell. However, overeating causes a reduction in the number of leptin receptors leading to leptin resistance and a further increase in food intake. Another molecule released by fat cells which have implications for insulin resistance is lysophosphatidic Acid (LPA).
LPA is a bioactive lipid that regulates various physiological conditions, such as vasoregulation, chemotaxis, cell growth and survival. The enzyme autotaxin (ATX), produced by fat cells, is the major determinant of serum LPA. Aberrant production of LPA, however, has been associated with impaired glucose homeostasis and obesity-induced insulin resistance.
How does Diet affect LPA?
Serum concentration of LPA is regulated by fasting and dietary intake. In mice, the circulating levels of ATX-LPA levels were significantly decreased by about 50% following 16 hours fasting, whereas higher levels were detected in fed state. Following feeding, LPA induces the differentiation of adipocyte and pre-adipocyte, as well as decreased glycogen storage in the body. Macronutrients (fats, proteins and carbohydrates) have also been shown to regulate ATX-LPA expression, in particular mice fed high-fat diet or obesogenic diet; a high-fat- high-sucrose diet (45% kcal, 17% kcal sucrose) induced a 62% increase in serum levels of plasma LPA 2.
It there a link between LPA and obesity?
Adipocyte hypertrophy and hyperplasia are both regulated by the ATX-LPA axis through autocrine and paracrine signalling. The ATX-LPA signalling pathway also plays an essential role in recruiting premature fat cells (pre-adipocytes), altering adipose tissue biology and expanding adipocyte fat mass. In vivo, extensive reports in murine models have shown ATX-LPA axis plays an important role in nutritional fatness and obesity.
However, it is not clear whether obesity induces increased LPA expression or vice-versa. A study has shown that feeding a high-fat diet to mice with and without the ATX gene causes an increase in adipocyte fat size suggesting adipose hypertrophy at least can be signalled without ATX-LPA axis 3,4. In humans, however, a recent report shows that LPA concentration in serum directly correlates with BMI6. Serum ATX concentration has also been shown to correlate with waist circumference and BMI of obese and overweight patients7.
How does an increased level of LPA affect insulin resistance and glucose homeostasis?
ATX-LPA levels in human serum correlate with several insulin sensitivity and glucose homeostasis markers, such as glucose infusion rate, fasting blood sugar and insulin resistance. The protein concentration in serum predicts glucose homeostasis is in relation to age and obesity5.
Also, ATX mRNA levels in the abdominal adipose tissue of obese women who exhibited impaired glucose intolerance were significantly higher when compared to control. In animal studies, ATX-LPA axis is implicated in the regulation of obesity glucose homeostasis. Exogenous injecting of a supraphysiological dose of LPA induced acute glucose tolerance in both chow and high fat fed mice 4. This suggests a direct role for ATX-LPA in regulating glucose homeostasis and insulin resistance. Clinical evidence of LPA signalling T2DM remain speculative and understanding of the signalling pathway of LPA could help elucidate the signalling mechanism between obesity and T2DM.
Currently, several PhD and Biomedical MSc students in the Chester Medical School are conducting studies aiming to understand the mechanism by which insulin resistance is induced. For example, we are investigating the effect of the anti-obesity supplement: Conjugated linoleic acid on insulin resistance and diabetes markers in obese and overweight women.
Another study is looking at the effect of probiotic-derived from the fermented milk on insulin resistance markers and other metabolic markers. Additionally, a PhD project is looking at the concentration of serum LPA in T2DM patients compared to healthy volunteers and how it correlates with insulin resistance and other diabetic markers.
Chester Medical School is not only keen to contribute in establishing several T2DM types of research but also, we are running the MSc Diabetes and MSc Cardiovascular Disease programmes. These two programmes aim to familiarise our medical students from various professional backgrounds with the current and novel pharmacological and non-pharmacological treatment options for T2DM and its associated CVDs complications. One important approach of these two programmes – in common with other Chester Medical School MSc programmes is to engage our medical school students in evidence-based practice to embed research in the mind of the clinical practice and to encourage proposing the novel management and treatment strategies for T2DM and its associated complications.
Please note: this is a commercial profile
References
[1] Prescribing for Diabetes England 2005-06 to 2013-14 – Health and Social Care Information Centre, 2014.
[2] Dusaulcy R., Rancoule C., Gres S., Wanecq E., Colom A., Guigne C., van Meeteren L.A., Moolenaar W.H., Valet P., Saulnier-Blache J.S. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J. Lipid Res. 2011;52:1247-1255. doi: 10.1194/jlr.M014985.
[3] D’Souza, K., Nzirorera, C., Cowie, A. M., Varghese, G. P., Trivedi, P., Eichmann, T. O., …Kienesberger, P. C. (2018). Autotaxin-Lysophosphatidic Acid Signaling Contributes to Obesity-Induced Insulin Resistance in Muscle and Impairs Mitochondrial Metabolism. J Lipid Res. doi:10.1194/jlr.M082008.
[4] Rancoule, C., Dusaulcy, R., Treguer, K., Gres, S., Attane, C., & Saulnier-Blache, J. S. (2014). Involvement of autotaxin/lysophosphatidic acid signaling in obesity and impaired glucose homeostasis. Biochimie, 96, 140-143. doi:10.1016/j.biochi.2013.04.010.
[5] D’Souza, K., Paramel, G. V., & Kienesberger, P. C. (2018). Lysophosphatidic Acid Signaling in Obesity and Insulin Resistance. Nutrients, 10(4). doi:10.3390/nu10040399.
[6] Michalczyk A., Budkowska M., Dolegowska B., Chlubek D., Safranow K. Lysophosphatidic acid plasma concentrations in healthy subjects: Circadian rhythm and associations with demographic, anthropometric and biochemical parameters. Lipids Health Dis. 2017;16:140. doi: 10.1186/s12944-017-0536-0.
[7] Reeves V.L., Trybula J.S., Wills R.C., Goodpaster B.H., Dubé J.J., Kienesberger P.C., Kershaw E.E. Serum Autotaxin/ENPP2 correlates with insulin resistance in older humans with obesity. Obesity (Silver Spring) 2015;23:2371–2376. doi: 10.1002/oby.21232.
Professor John Williams
Associate Dean of the Faculty of Medicine, Dentistry and Life Sciences
Prince Asiamah
PhD student at Chester Medical school
Hanady Hamdallah
Medical Science Lecturer
Chester Medical School
Tel: +44 (0) 1244 513 860 (Ext 3860)