Here, we discover the World Health Organization perspective on cannabidiol (CBD), including how there has been a shift in how CBD is socially perceived following reports and recommendations
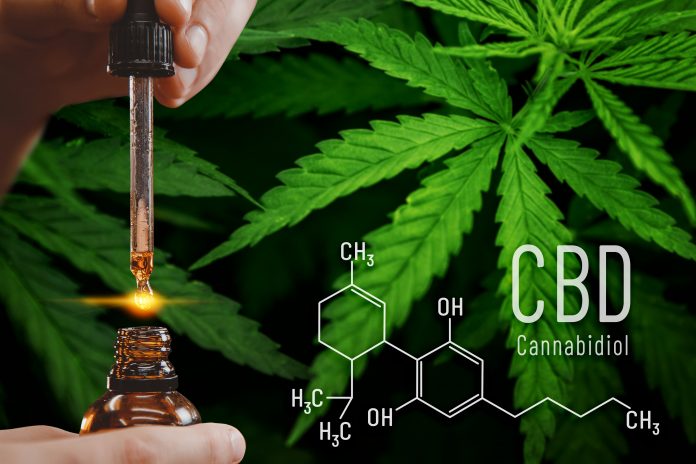
Cannabidiol, or CBD as it is generally known, is one of the main active compounds found in the cannabis plant. Unlike Tetrahydrocannbinol (THC) – the other main active compound in the cannabis plant — CBD is not psychoactive so using it does not result in the high associated with cannabis. As CBD is considered an extract of the cannabis plant, there is not the same international controls around its production and supply to the market as there is for the cannabis plant itself.
CBD studies
In November 2017, the WHO Expert Committee on Drug Dependence (ECDD) came to the decision that CBD “does not appear to have abuse potential or cause harm.” In animal studies and in controlled human studies, physical dependence on CBD has not been identified. For example, in studies with mice, there were no tolerance or withdrawal effects found. Similarly, it does not appear to have a stimulus, intoxication, physiological or psychotic effects.
For example, “an orally administered dose of 600mg of CBD did not differ from placebo on the scales of the Addiction Research Centre Inventory, a 16 item Visual Analogue Mood Scale, subjective level of intoxication or psychotic symptoms.” In contrast to the dopamine release in cells that occurs with most drugs of abuse, during animal testing CBD showed no such release. As well as this, THC use has been connected to anxiety and increased heart rate, but these symptoms have not been found in volunteers in CBD trials.
CBD market
Following on from this, in December 2017, WHO officially recommended that CBD should not be “internationally scheduled as a controlled substance.” As a result of these findings and recommendations by WHO, many countries including the United Kingdom, the United States of America, Canada and Australia have all relaxed regulations around CBD.
In line with these reports and recommendations, there has been a shift in how CBD is socially perceived, and the market for CBD products is steadily growing. In fact, there are now hundreds of thousands of regular CBD users in the United Kingdom alone. CBD is readily available on the market, both in shops and online and consumers can buy it in different forms, such as an oil to be orally consumed, supplements, gums or as an e-liquid for e-cigarettes and vapes.
CBD and epilepsy
There is currently no evidence that recreational use of CBD is connected to any health problems. In fact, it is considered to have many health benefits and is used to treat many health difficulties including stress, sleep difficulties, anxiety, other mental health difficulties, and chronic pain. The New England Journal of Medicine has run controlled studies on both humans and animals that suggest CBD has a valuable therapeutic use for epilepsy, seizures and spasms.
While more research needs to be done, there is already some evidence that CBD has medical use for calming spasms, during epileptic seizures and even limiting seizures altogether. In a small double-blind placebo trial, four patients were given 200mg of CBD a day and four patients were given a placebo for three months, which they took on top of their usual medication. Two of the patients trialling CBD saw improvements with no seizures during the time period, which one partially improved and one saw no change. Whereas, “no improvements were observed in the placebo group.”
In this study, as well as others, no toxic effects or serious side effects were noted. In another double-blind placebo-controlled trial, CBD was used as part of treatment for Dravet syndrome which is “a complex childhood epilepsy disorder that is associated with drug-resistant seizures and a high mortality rate.”
In the patients who took CBD along with their other treatment, the medium frequency of convulsive seizures in a month fell from 12.4 to 5.9 and 5% of patients had no seizures. This contrasts with the results of the patients taking the placebo, where the medium frequency of seizures fell from 14.9 to 14.1 and no patients experienced any seizures. However, the side effects from the CBD were experienced at a higher rate in patients taking CBD than patients taking the placebo. These side effects included diarrhoea, loss of appetite, somnolence, vomiting and fatigue.
The research into CBD and epilepsy is more advanced than the use of CBD to treat other medical conditions but there is some evidence – both pre-clinical and clinical – that CBD could have “neuroprotective, antiepileptic, hypoxia-ischemia, anxiolytic, antipsychotic, analgesic, anti-inflammatory, anti-asthmatic, and antitumor properties.” Therefore, in the future, CBD may have therapeutic benefits for a range of medical conditions from arthritis to depression.
References
CANNABIDIOL (CBD) Critical Review Report Expert Committee on Drug Dependence Fortieth Meeting Geneva, 4-7 June 2018 https://www.who.int/medicines/access/controlled-substances/CannabidiolCriticalReview.pdf
CANNABIDIOL (CBD) Pre-Review Report Agenda Item 5.2 Expert Committee on Drug Dependence Thirty-ninth Meeting Geneva, 6-10 November 2017 https://www.who.int/medicines/access/controlled-substances/5.2_CBD.pdf
The health and social effects of nonmedical cannabis use https://www.who.int/substance_abuse/publications/msbcannabis.pdf (ISBN 978 92 4 151024 0)











There has been no relaxation, nor change of any sort concerning CBD in the UK. It is not a controlled drug and never has been. In fact there are no regulations, nor laws specifically about CBD in the UK at all.
[…] to the perspective of the World Health Organization, there is no indication that the use of CBD has been associated with any public health problems. […]