Professor Luis Diestre discusses the research and findings of his European Research Council funded Illegal Pharma Project, focussing on the ever popular market for counterfeit pharmaceuticals
The sale of counterfeit drugs through illegal online pharmacies has grown dramatically in the most recent years. Worth $400 billion a year, the market for counterfeit pharmaceuticals surpasses almost every other illegal activity. The public health consequences are disheartening. Because illegal pharmacies often supply substandard medicines, the WHO estimates that up to 1 million people (250,000 being children) die annually from counterfeited drugs (World Health Organization, 2018).
Health regulatory authorities from affected countries, together with the Interpol, have intensified their activities to stop the trade of illegal medicines. For instance, operation Pangea, a coordinated effort among 123 countries, led to the seizure of more than 25 million counterfeit medicines and 3,583 websites taken offline in 2017. However, in spite of these global efforts, illegal online pharmacies continue to thrive. The reason, apparently, is that there is still a strong demand for counterfeit drugs. 1/3 of the participants of a survey conducted in the US responded that they have used an illegal online pharmacy to purchase medications for themselves, a family member, or someone under their care (ASOP Global, 2017). Thus, while demand for illegal medicines persists, criminal organisations will have an incentive to offer these products.
Law enforcement attempts to tackle this fundamental problem from the supply side of the market, i.e., limiting the offering of counterfeit drugs. Yet, there is little being done to address the demand side of the problem, i.e., customers’ willingness to acquire counterfeit medicines. The reason may be that we have a very limited understanding about why people are willing to take counterfeit medicines and put their health at risk. The overly accepted idea that counterfeit medicines are cheaper provides an incomplete, if not misleading answer to this issue. First, it does not explain why certain medicines, but not others, are offered in illegal online pharmacies. Second, while on average counterfeit drugs are cheaper, many counterfeit drugs are actually more expensive than their legal versions. All this suggests a more complex picture about the nature of the demand for counterfeit medicines. The goal of Professor Diestre’s ERC-funded ILLEGALPHARMA project is precisely to address this gap as a way to help regulatory authorities in their fight against this urgent challenge.
ERC project goals
In a nutshell, Professor Diestre’s ERC Starting Grant tries to answer the following three fundamental questions:
- Why are certain medicines offered in illegal pharmacies but not others?
- Why are illegal medicines cheaper than their legal versions in some cases but more expensive in others?
- Why are illegal firms able to capture a large market share in certain markets but a small market share in others?
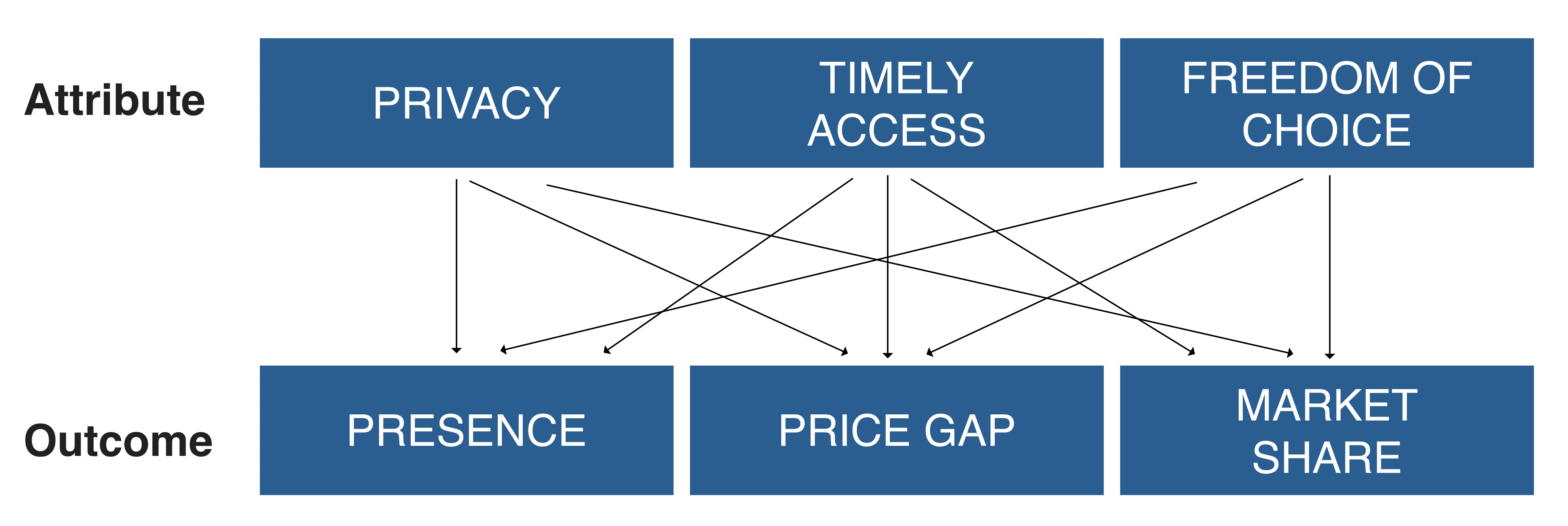
To answer these questions, this project explores the reasons why customers are likely to find counterfeit products more attractive than the legal versions. Specifically, this project focuses on three fundamental attributes that may increase customers’ preference for counterfeit drugs: privacy, freedom of choice, and accessibility.
What are the main propositions and findings of the project?
Privacy: The fact that patients do not need to provide a prescription to acquire counterfeit medicines means that there is no need to visit a doctor or share information with one’s insurance provider, and information about the particular condition and medication will not appear in the patient’s records. This suggests that patients who value privacy (e.g., those suffering conditions that are highly stigmatised or that could significantly increase their insurance premiums) are likely to have a greater preference for counterfeit drugs. Consistent with this, it is quite common to find drugs like aripiprazole and sildenafil, which treat highly stigmatised conditions such as mental disease or erectile dysfunction respectively, at higher prices than their legal versions.
Timely access: Because illegal online pharmacies face significantly greater flexibility in their logistics and manufacturing processes (i.e., they do not need to follow manufacturing and logistic restrictions arising from regulatory monitoring and control) they are less likely to run out of stock than legal pharmacies. Therefore, illegal online pharmacies are likely to enjoy a competitive advantage within customers that suffer diseases that require a stable consumption and thus significantly value timely access. This is likely to explain why drugs with strong withdrawal effects, such as those treating insomnia like flurazepam, are frequently found in illegal pharmacies.
Freedom of choice: While customers buying from legal sources may face certain restrictions when trying to take medicines for off-label uses, these restrictions do not apply to illegal pharmacies. Thus, illegal online pharmacies are likely to enjoy a competitive advantage within customers that want to use drugs for off-label conditions and thus value freedom of choice. This may explain why drugs like misoprostol, approved to treat gastric ulcer but used off-label for termination of pregnancy, are frequently offered in illegal online pharmacies.
Project implications
This project has important implications for both drug manufacturers and regulatory authorities. On the one hand, it suggests that pharma companies should broaden the way they think about their competitive landscape. Illegal pharmacies represent a unique type of competitor with particular advantages (and disadvantages). Figuring out how to address patients’ needs better than illegal competitors may proof highly useful to avoid losing market share to these illegal companies. On the other hand, this project offers useful insights to regulators. Mainly, it suggests which drug-markets are more likely suffer the threat of counterfeit versions, which may allow regulators distribute their efforts and resources more efficiently in their fight against this growing challenge.
Illegal Pharma project has received funding from the European Union’s HORIZON 2020 Research programme under the Grant Agreement no. 715536.
Please note: This is a commercial profile