Here, Professor of Biochemistry, Microbiology & Immunology at the University of Saskatchewan, Peter Betscher, explains how he is dedicated towards realising Robert North’s vision
Several forms of cancer immunotherapy have been recently developed. They are of proven promise and very exciting. The underlying strategies exploit our understanding that immunity is naturally generated against cancers. They seek to enhance the generation of, or to create, protective, cytotoxic T lymphocytes (CTL), able to kill cancer cells.
I suggest the unprecedented increase in immunological information at the molecular and cellular levels has perforce led to a neglect of older observations and considerations at the level of the system. Prominent among these are Robert North’s on tumour immunology, published in the 1980s. North addressed two questions at the level of the system: do tumours induce protective immunity and, if so, why are they not rejected? In general, valid considerations/observations at the level of the system provide a context for appreciating the significance of cellular/molecular considerations and observations. Invalid ideas at the level of the system provide an invalid context.
North was clearly inspired by the possibility of realising cancer immunotherapy. His motivation came from the existence of “concomitant tumour immunity”, as I shall describe. North’s approach and findings greatly influenced me. It is a propitious time to consider how recent, innovative forms of immunotherapy can be placed in the broader picture, developed by North and earlier investigators.
This melding should pave the way for even more effective cancer therapy. I first summarise the recent advances in cancer immunotherapy. I then outline older studies on protective immunity against animal tumours and its regulation. Lastly, I speculate on what prospects might arise from such a melding.
Modern strategies of immunotherapy
Checkpoint blockade
Activated T cells, including CTL, express surface antigens not expressed by naïve T cells. Among these are CTLA-4 and PDI that, on interacting with their ligands, generate feedback, inhibitory signals on the further activation of the T cell. Blocking these feedback interactions, referred to as “checkpoint blockade”, constitutes dramatic treatment of cancer in several clinical settings. One drawback is the variability in efficacy. Another is that considerable inflammatory, autoimmune side-effects may occur, particularly with blockade of CTLA-4. This is altogether not surprising as CTLA-4-deficient mice die at a young age from autoimmunity.
Chimeric antigen receptors
Researchers have transfected into a patient’s T cells expression vectors, coding for “chimeric antigen receptors”, and then given these T cells back to the patient as treatment. These receptors allow the transfected T cells to interact with antigens expressed on cancer cells and to give rise to CTL able to kill them. This approach has resulted in some spectacular results. However, the approach is somewhat invasive and expensive. It does not attempt to harness the patient’s protective immunity and, for this reason, is not further discussed here.
Classical studies
Vaccinating animals against transplantable tumours
A normally lethal dose of tumour can be implanted into the skin and excised nine days later. This excision prevents progressive tumour growth. Such excised mice resist a normally lethal challenge about a month after excision. Adoptive transfer of protection by lymphocytes but not by serum shows this protection to be due to cell-mediated immunity and is tumour-specific. Susceptibility in other studies was shown to be often associated with a predominant antibody response. George Klein wrote in 1968 of vaccination: “It will be important…to achieve the objective…a stimulation of cell-mediated rejection with minimal risk of antibody enhancement.” These insights are at the level of the system.
Concomitant immunity
An animal given a lethal dose of a tumour, usually in the skin, is often able to reject a similar challenge at a second site implanted nine days after the first. This “concomitant immunity” is tumour-specific and due to protective T cells, as demonstrated by adoptive transfer. Despite this, the initial tumour implant grows progressively, for reasons to be shortly discussed.
North’s and our related studies
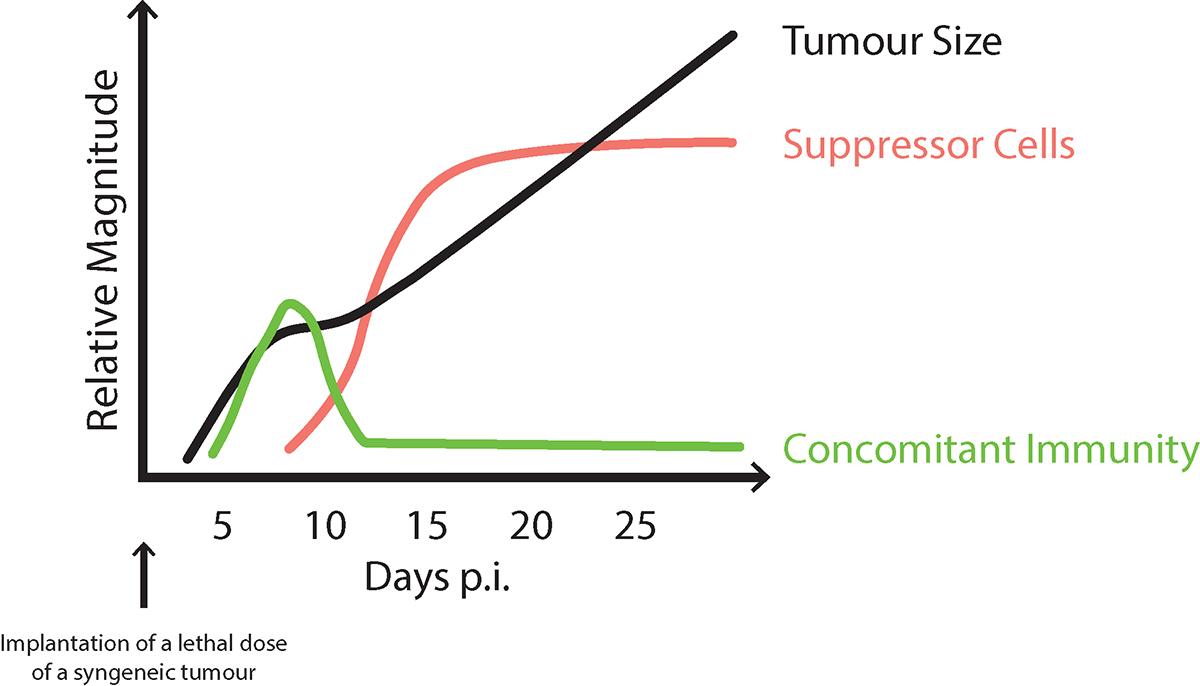
North was convinced that understanding how concomitant immunity is regulated would provide clues for effective immunotherapy. He showed that mice, implanted with a lethal dose of tumour, first generate protective CTL (concomitant immunity), but this generation declines as tumour-specific suppressor CD4 T cells appear, leading to progressive tumour growth, see Figure 1. North examined responses to several tumour types and uncovered the same pattern. His solution to the paradox of concomitant immunity and progressive tumour growth was: “too little (concomitant immunity), too late.”
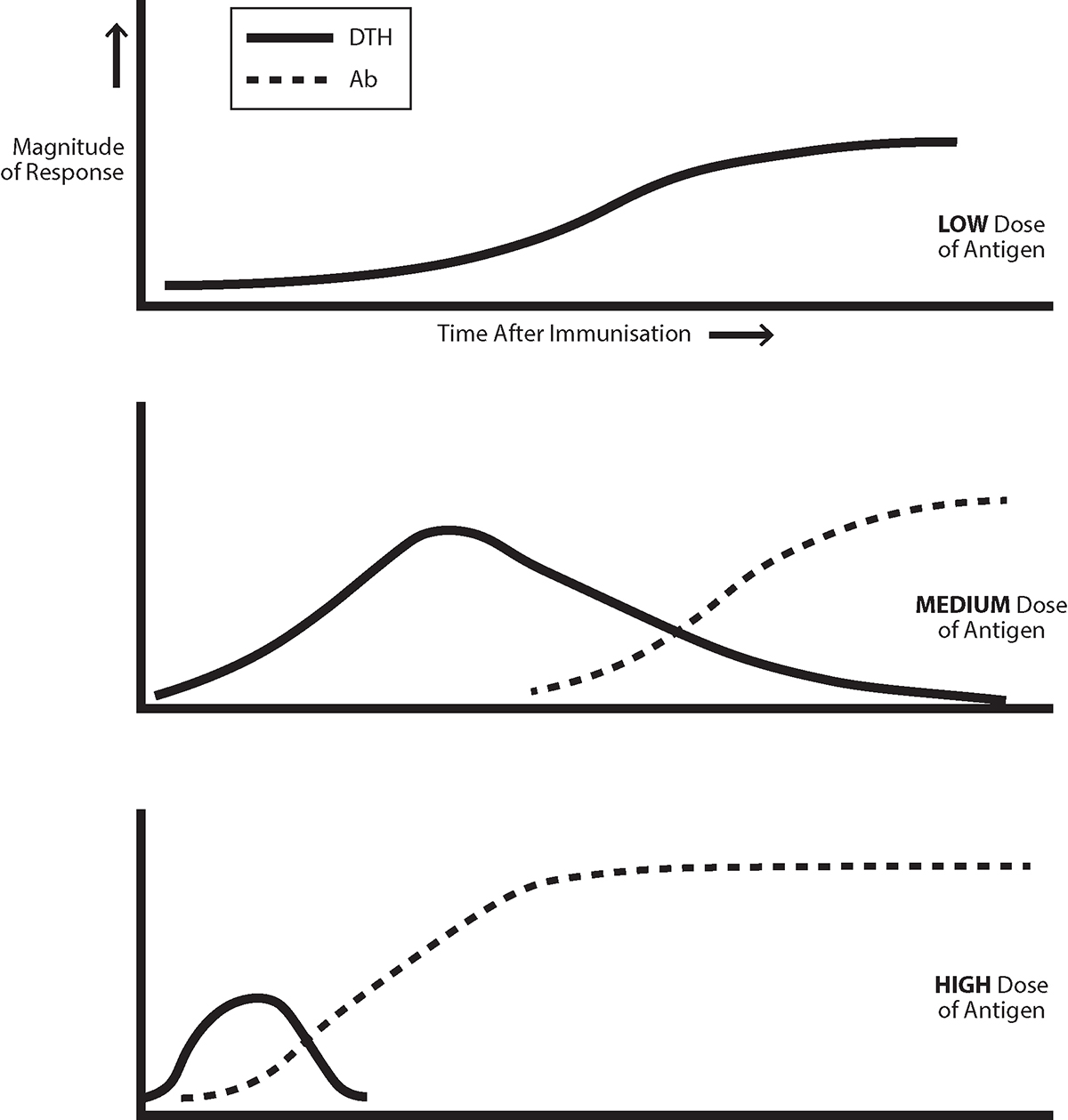
The pattern of response uncovered by North seems “familiar.” Antigen most often first induces a cell-mediated response, that is down-regulated as IgG antibody is produced and Th2 cells are generated, though a sufficiently low dose of antigen only generates a cell mediated, Th1 response, see Figure 2. We showed that lethal challenges of two tumours North employed first generate an exclusive Th1 response. The response evolves to gain an increasing Th2 component, with increasing tumour-specific IgG antibody production, and with the ratio of IgG1/IgG2 isotypes also increasing. As discussed previously in Open Access Government, similar patterns are seen in most people infected by HIV-1 and in the response of mice to, for example, Leishmania major.
Repetitive administration of appropriately low doses of a protein antigen not only induces exclusive cell-mediated immunity. It can guarantee such a response on a subsequent challenge with a high dose that results in antibody in naïve animals, as Parish showed in the late 1960s. We found infection with low numbers of L major parasites generates a Th1 imprint, and so resistance to a challenge with a high number that generates a predominant Th2 response and disease in naïve mice. We carried out similar experiments with tumours. These observations are the basis of the “low dose vaccination strategy.” This framework also provides a simple explanation for the efficacy of excision priming to achieve tumour resistance. The tumour is removed at a time when cell-mediated immunity is optimal; its removal undermines the evolution of the response towards a Th2 mode.
Improving Cancer Immunotherapy
As noted above, a low antigen dose leads to an exclusive, primary Th1 response. We demonstrated that deceasing the antigen load in patients with an on-going Th1/Th2 response can modulate the response towards a Th1 mode. The decrease in load must be appropriate; we argue that, if the decrease is too great, all immunity is lost. An appropriate decrease is one that results in a predominant Th1 response, associated with predominant IgG2 antibody production, as discussed elsewhere, see Open Access Government.
Perhaps much human cancer is associated with ineffective immunity containing a detrimental, Th2 component, associated with production of cancer specific IgG1 antibodies. Surgery and chemotherapy reduce the antigen load. Thus, such treatments have the potential to increase protective immunity if the drop in antigen load is appropriate in degree. This briefly indicated protocol parallels one, described elsewhere, see Open Access Government, for treating HIV-infections. An examination of the levels of different IgG isotypes, among anti-cancer antibodies, will likely be useful as an indicator whether checkpoint blockade will be effective. It may well be that therapy is ineffective if immunity contains a significant Th2 component. The confirmation of such a correlation would provide a context for designing combined therapy, such as chemotherapy, and checkpoint blockade when the response has been modulated to a protective, predominant Th1 mode.
A very recent publication (Frontiers in Immunology, 2021 12, article 764596) that appeared since this was drafted, demonstrates that resected samples of primary, human lung tumours show a predominance of infiltrating Th2 cells. I find this very exciting.
Please note: This is a commercial profile.
© 2019. This work is licensed under CC-BY-NC-ND.