Rick Clayton, Technical Director, AnimalhealthEurope, in the second chapter of this three-part series, continues to examine Benchmarking Regulatory systems governing veterinary medicines
This is the second in a series of three articles looking at Benchmarking Regulatory systems, using the regulatory systems governing veterinary medicines as a working example.
The first article looked at the importance of benchmarking regulatory systems around the world, and reported on the HealthforAnimals Global Benchmarking Survey 2020, covering 10 countries plus the EU.
This second article looks at key metrics and recommendations from the 2020 survey that are generally applicable to regulatory regimes for any regulated industry.
As an industry characterised by a high level of investment in research, benchmarking the competitiveness of the global animal health industry is of great importance. The HealthforAnimals Global Benchmarking Survey 2020 identified the following areas for key metrics:
- Impact of regulations on ability to innovate.
- Impact of regulations on ability to commercialise existing products, including the diversion of R&D budgets into “defensive R&D” (i.e. the cost of additional studies demanded by the regulatory authority to maintain a product on the market).
- Time and cost to market:
– Time for new product development.
– Time for product registration phase.
– The cost of new product development.
- The challenges going forward.
- Hopes and expectations of the industry.
A fundamental question underlying these metrics ties together the cost of market entry with the size of the market; is it possible to achieve a return-on-investment within an acceptable timeframe and with an acceptable level of business risk?
Key trends
The Global Benchmarking data confirm that companies appreciate a well-regulated market, but are negatively impacted by excessive or perhaps unnecessary aspects of the regulations. Negative company views may also reflect regulations that are unclear, complex or enforced in a disharmonised or unpredictable manner.
Companies’ regulatory strategies can vary considerably depending on their business model. For example – the allocation of R&D funding to support products already on the market. A small number of companies focus their funds almost entirely on developing new products and have decided no longer re-invest in repeatedly ‘re-developing existing products. Others invest heavily in maintaining the lynchpins of their product portfolio. Ultimately, the future of all animal health companies relies on a high level of investment in innovation.
The time and cost required for new product development projects are increasing, a long-term trend over several decades. As societal expectations evolve, public policy seeks to increasingly minimise risks to the animal patient, the product user (e.g. veterinarians), the consumer (of food) and the environment. This pushes up data requirements and the time needed to complete all the studies.
The length of the registration phase can vary tremendously between countries, often associated with the economic development of the country (Figure 1). The 2020 survey data illustrates a marked difference between countries, ranging from an average of 1.3 years to 6.4 years. Target deadlines for scientific reviews of marketing authorisation applications should be set in legislation.
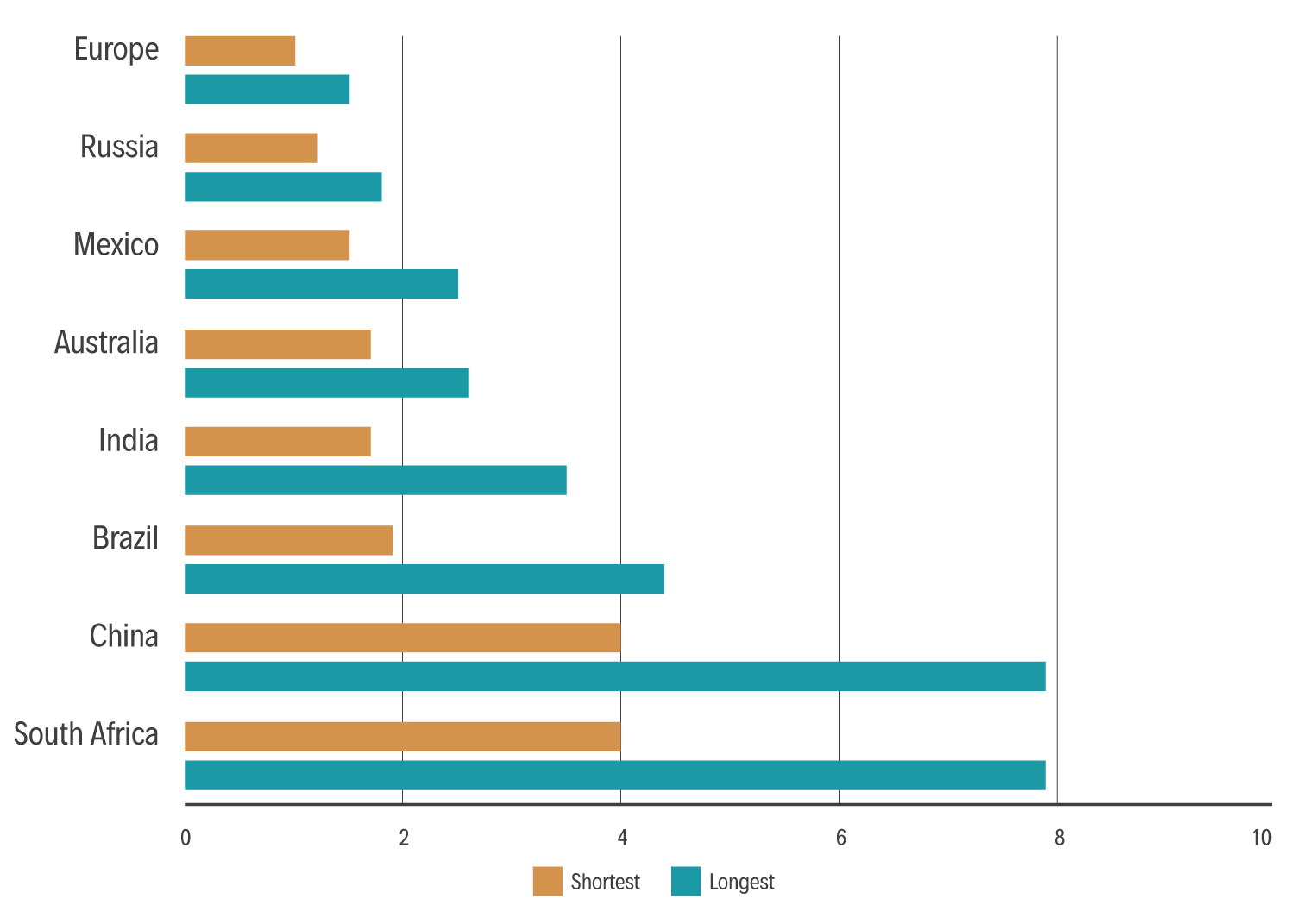
Marked differences between registration times may also exist for different product categories, such as between companion animal and food-producing animal medicines (where extra consumer and environmental safety studies are required), or between vaccines and pharmaceuticals (vaccines usually have a biological component, expanding the scientific review process).
An emerging trend is for some countries to offer a faster registration process for products that are already registered in a country with a ‘stringent’ regulatory authority working to international standards, e.g. biologicals can be approved in six months in Canada if already approved in the U.S.
Although the rate of increase of product development costs has slowed down in developed countries, it has risen significantly in transition countries that are upgrading their regulatory systems to international standards.
The challenges
Several key challenges were identified when comparing regulatory systems across the 11 markets:
- Suitable periods of intellectual property protection, essential to support investment, are not always present.
- Predictability of the regulatory processes is critical and is sometimes lacking.
- Product development requires a long-term investment. The longer the time-to-market, the higher the risk to the business plan and obtaining a return on investment.
- In some countries, companies face unannounced or changing requirements, poor communication with regulatory agencies, and long and unpredictable timelines. This is often caused by insufficient resources allocated to regulatory agencies, resulting in understaffing, lack of sufficient training and lack of investment in regulatory infrastructure, including digital tools.
- The trend towards increasing cost and time required for product development has been persistent for decades. This creates a need to spread the cost and risk across global markets and raises the importance of global regulatory convergence, particularly for smaller national markets.
- New data requirements applied to existing products mean high defensive R&D costs persist. Other post-authorisation costs are also increasing with the implementation of Good Manufacturing Practice requirements and improved pharmacovigilance systems.
The future and suggestions for action
The Global Benchmarking reports recognise the progress that has been made in modernising regulatory frameworks. Although well advanced in some countries, progress is in its infancy in others.
The modernisation of regulatory frameworks should be seen as an opportunity to better adapt systems to the specific characteristics of the veterinary sector; for example, by avoiding lengthy regulatory pathways involving several government ministries.
Countries should move towards regulatory convergence with the adoption of international standards and participation in international regulatory harmonisation initiatives. Regulatory science must also adapt to new scientific approaches and novel technologies to encourage and not hinder innovation and investment.
Participation in international initiatives and alignment with international standards helps build trust and mutual respect, facilitating resource-efficient approaches such as joint assessments, work-sharing, recognition of decisions of trusted agencies and reduced duplication of inspections and audits.
The ability to respond rapidly to new emerging disease threats requires an agile and flexible regulatory system. Therefore, greater emphasis should be placed on nuanced regulatory approaches, such as conditional approvals, fast-track approvals and mutual reliance on decisions taken in other trusted jurisdictions.
Finally, more emphasis is needed on the application of the principles of the “3Rs” in the reduction of the mandatory use of animals in regulatory science, both at the product development stage and for routine batch release.











You make an excellent point that it is important for us to choose a qualified animal hospital for our pets.
My wife and I would like to have our dogs checked. I will look into reliable hospital for our pets.