Jason Tait Sanchez, Assistant Professor at Northwestern University provides insight into why the genetically modified chicken is a sound approach to the study of hearing. We discover how tonotopic properties are established in the chicken auditory brainstem by using novel and innovated genetic research methods
It is well established that the chicken is a valuable research tool to study basic biological questions in numerous health-related areas, including immunology and infectious diseases(1,2).
Recent applications of gene editing in chickens also suggests an innovative era is on the horizon for developmental and sensory neurobiology as well(3,4). With respect to hearing development, mammals and birds share comparable auditory functions at the cellular, synaptic, and neural circuit level(5,6) and both species encode sound similarly across the frequency axis, a process known as tonotopic tuning(7,8).
Tonotopy is the spatial arrangement of where sounds of different frequencies are processed. Tonotopy originates along the peripheral sensory epithelium and is preserved throughout the entire auditory system. Tonotopy in the central auditory pathway is arranged not only by the specific locations of neurons and their inputs, but by differences in their structural and functional properties along the tonotopic axis(9,10). Exemplars of this are found in the mammalian anteroventral cochlear nucleus and the chicken cochlear nucleus magnocellularis (NM), which are analogous, first-order auditory brainstem structures. In this article, I will provide recent insight into how tonotopic properties are established in the chicken auditory brainstem by using novel and innovated genetic research methods.
Development of tonotopic properties
Despite more than a half-century of work on the development of tonotopic properties in the peripheral auditory system (i.e., the cochlea), little is known about its establishment in the central auditory system(11). This fundamental lack of knowledge is noteworthy, and several questions warrant discussion.
First, do tonotopic properties emerge from indiscriminate connections, or are there precise projections early in development? If precision exists early on, does refinement improve with maturation? Anatomical evidence argues that the topography of connections between the periphery and central pathway develops with considerable precision, well before hearing onset, and with substantial refinement thereafter(12-18).
Second, what role, if any, does spontaneous neuronal activity – as opposed to sound-driven activity – have on the development or maintenance of tonotopic properties? Physiological studies show that early in development, functional mapping along the tonotopic axis supports precise tuning independent of sound-driven activity (19-23).
Finally, what are the molecular and cellular signals responsible for establishing and maintaining distinct neuronal phenotypes along the tonotopic axis in the central pathway? The answer to this final question remains elusive, making it a significant and timely problem in developmental and sensory neurobiology in general(3).
Potential genes-of-interest
One thing is clear, however; both presynaptic axons and postsynaptic target neurons express genes – like neurotrophins – that may be responsible for establishing tonotopic properties in the central pathway. Neurotrophins, along with their cognate receptors, are growth factor proteins that support numerous aspects of normal nervous system development(24-27), and irregular neurotrophin signalling is implicated in pathophysiological conditions in both the peripheral and central nervous systems(28-30). This makes them a critical factor that promotes normal and abnormal biologically relevant properties(31).
The idea that neurotrophin signalling is important for the tonotopic establishment in the auditory system is supported by the following observations from the chicken NM.
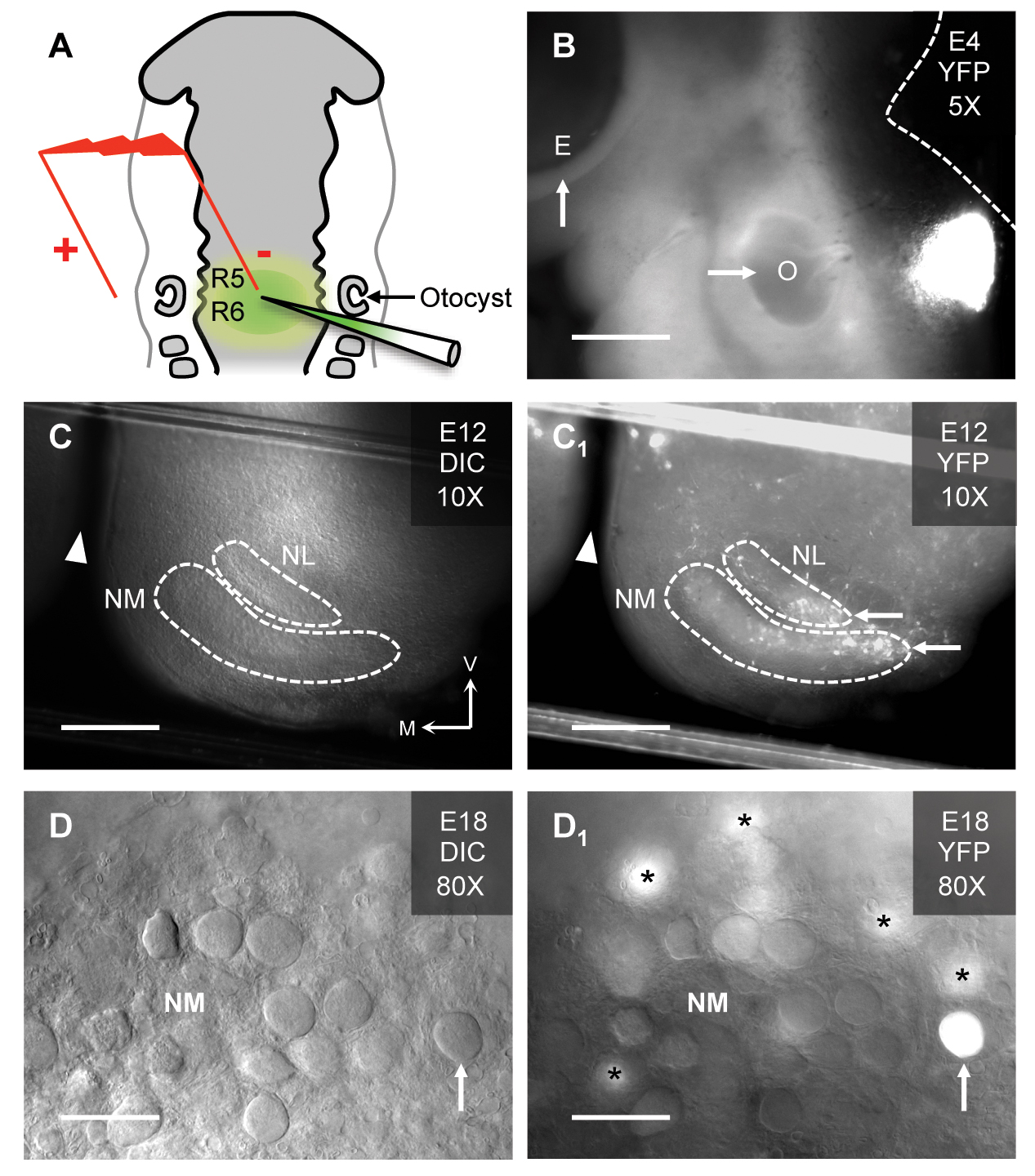
(A) Schematic of electroporation in the hindbrain of a Hamburger Hamilton (HH) stage 12 chicken embryo. The injection pipette is filled with plasmid DNA and fast green dye for visualisation of the injected hindbrain (rhombomeres 5/6 [R5/R6]). In this study, a plasmid that coexpressed the gene-of-interest and the yellow fluorescent protein (YFP) reporter was used. Electroporated embryos are further incubated until the desired developmental stage is reached. (B) Embryonic (E) day 4 chicken showing the site of YFP expression relative to the left eye (arrow, E) and left otocyst (arrow, O). The dashed white line represents the dorsal brain and brainstem border. Scale bar = 480 μm. (C & C1). Brainstem slice (300 μm thick) from an E12 chicken under differential interference contrast (DIC, C) and fluorescent (YFP, C1) illumination. Dashed white lines represent the borders of nucleus magnocellularis (NM) and nucleus laminaris (NL). Note, the brainstem slice shows only one side of the tissue. White arrowheads represent midline cleft of the brainstem slice. White arrows show YFP expressing regions of NM and NL. V = ventral, M = medial. Scale bar = 240 μm. (D & D1). High magnification (80X water immersion objective) of an E18 chicken brainstem slice containing NM. The classic adendritic cell bodies37 are clearly visible under DIC illumination (upward arrow, D). With fluorescent illumination for the same slice, a transfected NM neuron identified by YFP fluorescence is clearly visible (upward arrow, D1). Asterisks = YFP expressing NM neurons just below the focal plane. Scale bar = 30 μm. Figure from Lu et al., 201748.
Second, the expression pattern of a very specific neurotrophin receptor (known as TrkB) is spatially and temporally dynamic; TrkB is present at embryonic (E) day 7, significantly reduced by E15 and absent at hatch (E21), but only in mid- to high-frequency regions(33).
Third, this expression pattern parallels a developmental period when functional properties are also differentially established along the tonotopic axis(34-37) and coincides with hearing maturation(38).
Finally, genetically modified maintenance of TrkB receptors in mid- to high-frequency NM prevents dendrite retraction and promotes aberrant neuronal excitability(39), properties that more closely resemble their low-frequency neuronal counterparts(10,40). The dynamic expression pattern of TrkB receptors regulates the development of distinct tonotopic properties found in NM and strongly supports the hypothesis that neurotrophin signalling establishes different neuronal phenotypes along the tonotopic axis in the central auditory pathway.
Why the chicken?
The chicken is an ideal model system over other mammalian research tools because they have tonotopic properties more commonly shared with humans. Chickens, like humans, utilise both low- and high-frequency sounds to perform behaviourally relevant auditory tasks, such as sound localisation and signal discrimination(41,42). This is unlike most mammalian research models, such as mice and rats, which rely primarily on ultrasonic hearing.
With respect to the development of hearing acuity, chickens (like humans) are also precocious animals. The chicken’s auditory system is near functional maturity at hatch, and the onset and refinement of hearing occur during embryonic stages38. This is unlike other low-frequency hearing mammalian research models (e.g., gerbil, guinea pig), whose hearing emerges ~10-16 days postnatal(43) and are susceptible to extrinsic factors that influence development.
Finally, the spatial and temporal expression of limited neurotrophin factors in the chicken NM(33,44) provides a novel opportunity to evaluate highly-specific neurotrophin signalling and its role in establishing neuronal topology. This is unlike the mammalian auditory system, which expresses many more neurotrophin factors across numerous developmental periods(45), ultimately confounding the study of neurotrophin signalling in regulating tonotopic development in these species.
A sound approach
Electroporation is a method that introduces genes into biologically relevant organisms like the chicken embryo. In ovo electroporation is a formidable tool to study neuron-specific development in the auditory brainstem(3,46). It permits the over-expression or knock-down of specific genes-of-interest (like neurotrophin factors) in order to analyse in vivo gene function(39,47). We and others have recently introduced genetic methods to obtain focal, stable and temporally regulated transgene expression of neurotrophins at multiple stages of chicken embryo development(3,39,48) (Fig. 1). It is advantageous over mammalian model systems for several reasons. First and foremost, because electroporation is performed in ovo, it permits gene expression in a normally developing biological system.
Second, genes are focally injected, allowing spatial control of expression in highly specific brain regions(49). Third, genes are temporally regulated by drug applications, enabling expression at precise developmental time periods(39,48).
Finally, genes are only expressed by a subset of neurons, allowing non-transfected neurons to serve as internal controls. This provides a rigorous and quantitative comparison of the neuron-autonomous effects of gene expression. The in ovo electroporation technique – together with either biochemical, pharmacological, and or in vivo functional assays – provides a genetic approach to study auditory neuron development associated with tonotopic differences in neuronal structure and function, as well as associated pathophysiological phenomena.
Indeed, a better understanding of normal auditory circuit assembly – along with unique structural and functional properties associated with tonotopic gradients – will provide a significant foundation for developing stem cell-based therapies for auditory-related disorders. However, stem cell-derived auditory neurons will only prove useful – therapeutically – if they are able to re-create neuronal properties that are characteristic of normal circuit maturation(50). A careful characterisation of neurotrophin signalling, the underlying molecular mechanism by which it operates, the role it plays in establishing normal neuronal properties, and the functional consequence of altering this biological process is necessary and appropriate.
Our research aims at addressing these issues by providing a comprehensive understanding of neurotrophin signalling and its role in establishing neuronal phenotypes along the tonotopic axis in the developing auditory brainstem, a biologically relevant structure which is essential for sound processing.
References
1 Lyall, J. et al. Suppression of avian influenza transmission in genetically modified chickens. Science 331, 223-226, doi:10.1126/science.1198020 (2011).
2 Farzaneh, M., Hassani, S. N., Mozdziak, P. & Baharvand, H. Avian embryos and related cell lines: A convenient platform for recombinant proteins and vaccine production. Biotechnol J 12, doi:10.1002/biot.201600598 (2017).
3 Sid, H. & Schusser, B. Applications of Gene Editing in Chickens: A New Era Is on the Horizon. Frontiers in Genetics 9, doi:10.3389/fgene.2018.00456 (2018).
4 Dimitrov, L. et al. Germline Gene Editing in Chickens by Efficient CRISPR-Mediated Homologous Recombination in Primordial Germ Cells. PloS one 11, e0154303, doi:10.1371/journal.pone.0154303 (2016).
5 Koppl, C. Evolution of sound localisation in land vertebrates. Current biology: CB 19, R635-639, doi:10.1016/j.cub.2009.05.035 (2009).
6 Carr, C. E., Soares, D., Parameshwaran, S. & Perney, T. Evolution and development of time coding systems. Current opinion in neurobiology 11, 727-733 (2001).
7 Grothe, B., Pecka, M. & McAlpine, D. Mechanisms of sound localization in mammals. Physiological reviews 90, 983-1012, doi:10.1152/physrev.00026.2009 (2010).
8 Carr, C. E. & Soares, D. Evolutionary convergence and shared computational principles in the auditory system. Brain, behavior and evolution 59, 294-311 (2002).
9 Brew, H. M. & Forsythe, I. D. Systematic variation of potassium current amplitudes across the tonotopic axis of the rat medial nucleus of the trapezoid body. Hearing research 206, 116-132, doi:10.1016/j.heares.2004.12.012 (2005).
10 Wang, X., Hong, H., Brown, D. H., Sanchez, J. T. & Wang, Y. Distinct Neural Properties in the Low-Frequency Region of the Chicken Cochlear Nucleus Magnocellularis. eNeuro 4, doi:10.1523/eneuro.0016-17.2017 (2017).
11 Rubel, E. W. & Fritzsch, B. Auditory system development: primary auditory neurons and their targets. Annual review of neuroscience 25, 51-101, doi:10.1146/annurev.neuro.25.112701.142849 (2002).
12 Angulo, A., Merchan, J. A. & Merchan, M. A. Morphology of the rat cochlear primary afferents during prenatal development: a Cajal’s reduced silver and rapid Golgi study. Journal of anatomy 168, 241-255 (1990).
13 Friauf, E. Tonotopic Order in the Adult and Developing Auditory System of the Rat as Shown by c-fos Immunocytochemistry. The European journal of neuroscience 4, 798-812 (1992).
14 Fritzsch, B., Farinas, I. & Reichardt, L. F. Lack of neurotrophin 3 causes losses of both classes of spiral ganglion neurons in the cochlea in a region-specific fashion. The Journal of neuroscience: the official journal of the Society for Neuroscience 17, 6213-6225 (1997).
15 Willard, F. H. & Martin, G. F. The development and migration of large multipolar neurons into the cochlear nucleus of the North American opossum. The Journal of comparative neurology 248, 119-132, doi:10.1002/cne.902480109 (1986).
16 Schweitzer, L. & Cant, N. B. Development of the cochlear innervation of the dorsal cochlear nucleus of the hamster. The Journal of comparative neurology 225, 228-243, doi:10.1002/cne.902250208 (1984).
17 Schweitzer, L. & Cecil, T. Morphology of HRP-labelled cochlear nerve axons in the dorsal cochlear nucleus of the developing hamster. Hearing research 60, 34-44 (1992).
18 Snyder, R. L. & Leake, P. A. Topography of spiral ganglion projections to cochlear nucleus during postnatal development in cats. The Journal of comparative neurology 384, 293-311 (1997).
19 Lippe, W. & Rubel, E. W. Ontogeny of tonotopic organization of brain stem auditory nuclei in the chicken: implications for development of the place principle. The Journal of comparative neurology 237, 273-289 (1985).
20 Sanes, D. H., Merickel, M. & Rubel, E. W. Evidence for an alteration of the tonotopic map in the gerbil cochlea during development. The Journal of comparative neurology 279, 436-444, doi:10.1002/cne.902790308 (1989).
21 Lippe, W. R. Relationship between frequency of spontaneous bursting and tonotopic position in the developing avian auditory system. Brain research 703, 205-213 (1995).
22 Sterbing, S. J., Schmidt, U. & Rubsamen, R. The postnatal development of frequency-place code and tuning characteristics in the auditory midbrain of the phyllostomid bat, Carollia perspicillata. Hearing research 76, 133-146 (1994).
23 Ryan, A. F. & Woolf, N. K. Development of tonotopic representation in the Mongolian gerbil: a 2-deoxyglucose study. Brain research 469, 61-70 (1988).
24 Ibanez, C. F. Emerging themes in structural biology of neurotrophic factors. Trends in neurosciences 21, 438-444 (1998).
25 Schecterson, L. C. & Bothwell, M. Neurotrophin receptors: Old friends with new partners. Dev Neurobiol 70, 332-338, doi:10.1002/dneu.20767 (2010).
26 Schuman, E. M. Neurotrophin regulation of synaptic transmission. Current opinion in neurobiology 9, 105-109 (1999).
27 Arevalo, J. C. & Wu, S. H. Neurotrophin signaling: many exciting surprises! Cell Mol Life Sci 63, 1523-1537, doi:10.1007/s00018-006-6010-1 (2006).
28 Hu, Y. & Russek, S. J. BDNF and the diseased nervous system: a delicate balance between adaptive and pathological processes of gene regulation. Journal of neurochemistry 105, 1-17, doi:10.1111/j.1471-4159.2008.05237.x (2008).
29 Gupta, V. K., You, Y., Gupta, V. B., Klistorner, A. & Graham, S. L. TrkB receptor signalling: implications in neurodegenerative, psychiatric and proliferative disorders. Int J Mol Sci 14, 10122-10142, doi:10.3390/ijms140510122 (2013).
30 Singer, W., Panford-Walsh, R. & Knipper, M. The function of BDNF in the adult auditory system. Neuropharmacology 76 Pt C, 719-728, doi:10.1016/j.neuropharm.2013.05.008 (2014).
31 Bramham, C. R. & Messaoudi, E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Progress in neurobiology 76, 99-125, doi:10.1016/j.pneurobio.2005.06.003 (2005).
32 Rubel, E. W. & Parks, T. N. Organization and development of brain stem auditory nuclei of the chicken: tonotopic organization of n. magnocellularis and n. laminaris. The Journal of comparative neurology 164, 411-433 (1975).
33 Cochran, S. L. et al. Ontogenetic expression of trk neurotrophin receptors in the chick auditory system. The Journal of comparative neurology 413, 271-288 (1999).
34 Hong, H., Rollman, L., Feinstein, B. & Sanchez, J. T. Developmental Profile of Ion Channel Specializations in the Avian Nucleus Magnocellularis. Front Cell Neurosci 10, 80, doi:10.3389/fncel.2016.00080 (2016).
35 Lu, T. & Trussell, L. O. Development and elimination of endbulb synapses in the chick cochlear nucleus. The Journal of neuroscience: the official journal of the Society for Neuroscience 27, 808-817, doi:10.1523/JNEUROSCI.4871-06.2007 (2007).
36 Feng, J. J. & Morest, D. K. Development of synapses and expression of a voltage-gated potassium channel in chick embryonic auditory nuclei. Hearing research 216-217, 116-126 (2006).
37 Jhaveri, S. & Morest, D. K. Neuronal architecture in nucleus magnocellularis of the chicken auditory system with observations on nucleus laminaris: a light and electron microscope study. Neuroscience 7, 809-836 (1982).
38 Jones, T. A., Jones, S. M. & Paggett, K. C. Emergence of hearing in the chicken embryo. Journal of neurophysiology 96, 128-141, doi:10.1152/jn.00599.2005 (2006).
39 Schecterson, L. C., Sanchez, J. T., Rubel, E. W. & Bothwell, M. TrkB downregulation is required for dendrite retraction in developing neurons of chicken nucleus magnocellularis. The Journal of neuroscience: the official journal of the Society for Neuroscience 32, 14000-14009, doi:10.1523/JNEUROSCI.2274-12.2012 (2012).
40 Hong, H. et al. Diverse Intrinsic Properties Shape Functional Phenotype of Low-Frequency Neurons in the Auditory Brainstem. Front Cell Neurosci 12, 175, doi:10.3389/fncel.2018.00175 (2018).
41 Hyson, R. L. The analysis of interaural time differences in the chick brain stem. Physiology & behavior 86, 297-305 (2005).
42 Shannon, R. V., Zeng, F. G., Kamath, V., Wygonski, J. & Ekelid, M. Speech recognition with primarily temporal cues. Science 270, 303-304 (1995).
43 McFadden, S. L., Walsh, E. J. & McGee, J. Onset and development of auditory brainstem responses in the Mongolian gerbil (Meriones unguiculatus). Hearing research 100, 68-79 (1996).
44 Hallbook, F., Wilson, K., Thorndyke, M. & Olinski, R. P. Formation and evolution of the chordate neurotrophin and Trk receptor genes. Brain, behavior and evolution 68, 133-144, doi:10.1159/000094083 (2006).
45 Green, S. H., Bailey, E., Wang, Q. & Davis, R. L. The Trk A, B, C’s of neurotrophins in the cochlea. Anatomical record (Hoboken, N.J. : 2007) 295, 1877-1895, doi:10.1002/ar.22587 (2012).
46 Matsui, R., Tanabe, Y. & Watanabe, D. Avian adeno-associated virus vector efficiently transduces neurons in the embryonic and post-embryonic chicken brain. PloS one 7, e48730, doi:10.1371/journal.pone.0048730 (2012).
47 Chesnutt, C. & Niswander, L. Plasmid-based short-hairpin RNA interference in the chicken embryo. Genesis 39, 73-78, doi:10.1002/gene.20028 (2004).
48 Lu, T., Cohen, A. L. & Sanchez, J. T. In Ovo Electroporation in the Chicken Auditory Brainstem. Journal of visualized experiments: JoVE, doi:10.3791/55628 (2017).
49 Cramer, K. S., Fraser, S. E. & Rubel, E. W. Embryonic origins of auditory brain-stem nuclei in the chick hindbrain. Dev Biol 224, 138-151 (2000).
50 Appler, J. M. & Goodrich, L. V. Connecting the ear to the brain: Molecular mechanisms of auditory circuit assembly. Progress in neurobiology 93, 488-508, doi:10.1016/j.pneurobio.2011.01.004 (2011).
Please note: this is a commercial profile
Jason Tait Sanchez
Assistant Professor
Northwestern University
Tel: +1 847 491 4648











